Integer’s Expansion in Europe—5Qs at Medica/CompaMed 2022
By Sean Fenske, Editor-in-Chief | 11.14.22
With worldwide growth and capabilities, Integer offers speed to market and a reduction of supply chain complexity.
It’s been quite the hiatus since attending the Medica and CompaMed trade shows in Dusseldorf, Germany. My last visit to the event was 2019, prior to all the disruption created by the COVID-19 pandemic. With renewed enthusiasm, I was anxious to get back onto the show floor to meet with those companies attending the 2022 event. In anticipation of my visit, I reached out to a number of companies to get the scoop directly from them on what they are showcasing at the event, what challenges customers have brought them, and where they see their role within the industry in aiding medical device manufacturers. With that in mind, Fergus Higgins, senior director of product marketing—Cardio & Vascular at Integer, shared the following insights to help you determine if the firm should be a potential services partner for you in 2023 or beyond.
Sean Fenske: What technology or service are you emphasizing at Medica/CompaMed this year?
Fergus Higgins: Integer is committed to expanding our capacity and capabilities in Europe. In addition to our existing substantial footprint in Ireland, we recently announced a significant expansion of our New Ross, Ireland, guidewire manufacturing site. This follows recent announcements on the construction of a new site in Galway, Ireland, co-locating development and manufacturing for complex catheters, delivery systems, textiles, and complex braiding under one roof, as well as the acquisition of Aran Biomedical—a technology leader in medical textiles, complex braiding, and implant coatings. We look forward to discussing this and more at CompaMed.
Fenske: What’s the most common challenge customers inquire about and how do you address it?
Higgins: Two of the most common challenge areas our customers ask us to help solve are accelerating speed to market in developing new products and simplifying the complexity of their supply chains. Integer has co-located design and development innovation teams with manufacturing sites in global medical device hubs close to our customers. Our technology breadth and depth offer our customers single source with scale and capacity to reduce supply chain complexity and the number of suppliers with which they deal.
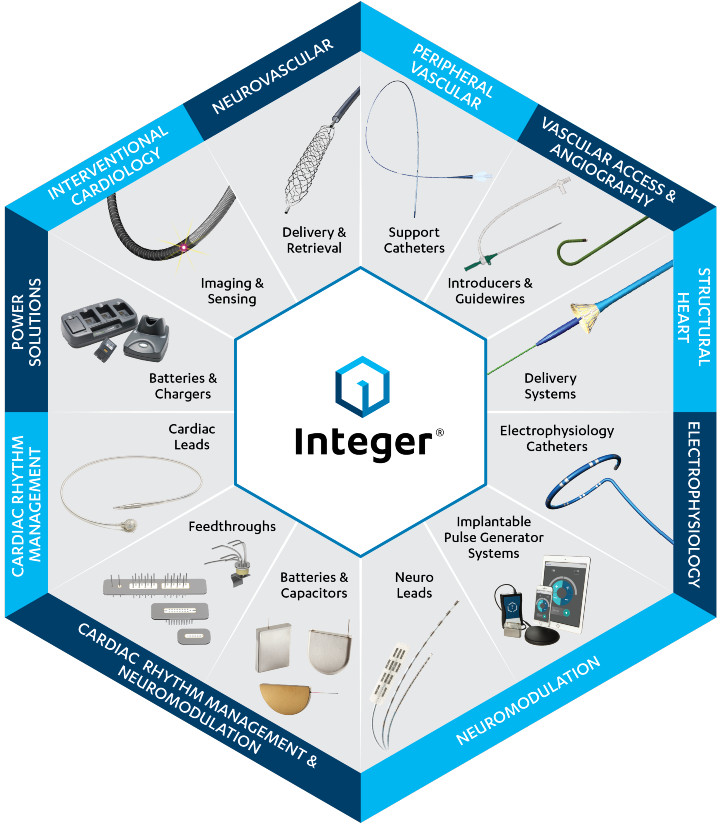
Integer offers an array of solutions for a variety of medical device segments.
Fenske: If you could give one piece of advice to companies seeking a manufacturing partner before they make a decision, what would it be?
Higgins: The one piece of advice we would give to companies seeking a manufacturing partner is to ensure the partner you choose has the size and scalability you require with a proven track record in quality of service.
Fenske: What are the forces driving medical device manufacturers to seek your technology/services over doing it in-house?
Higgins: Higher costs from ever-increasing regulatory standards coupled with global issues such as supply chain and labor shortages are major challenges for the market. Partnering with Integer can accelerate customers’ product development and increase speed to market, while access to our depth and breadth of technology furthers that competitive advantage.
Fenske: In what ways is your company able to aid in getting a product (project) to market faster?
Higgins: At Integer, our global project management organization (PMO) manages programs across our sites, leveraging our global quality system and intercompany supply of key components and technologies to accelerate development programs and achieve increased speed to market.
Integer is located at Medica/Compamed in Hall 8B, Booth/Stand P02.
Sean Fenske: What technology or service are you emphasizing at Medica/CompaMed this year?
Fergus Higgins: Integer is committed to expanding our capacity and capabilities in Europe. In addition to our existing substantial footprint in Ireland, we recently announced a significant expansion of our New Ross, Ireland, guidewire manufacturing site. This follows recent announcements on the construction of a new site in Galway, Ireland, co-locating development and manufacturing for complex catheters, delivery systems, textiles, and complex braiding under one roof, as well as the acquisition of Aran Biomedical—a technology leader in medical textiles, complex braiding, and implant coatings. We look forward to discussing this and more at CompaMed.
Fenske: What’s the most common challenge customers inquire about and how do you address it?
Higgins: Two of the most common challenge areas our customers ask us to help solve are accelerating speed to market in developing new products and simplifying the complexity of their supply chains. Integer has co-located design and development innovation teams with manufacturing sites in global medical device hubs close to our customers. Our technology breadth and depth offer our customers single source with scale and capacity to reduce supply chain complexity and the number of suppliers with which they deal.

Integer offers an array of solutions for a variety of medical device segments.
Fenske: If you could give one piece of advice to companies seeking a manufacturing partner before they make a decision, what would it be?
Higgins: The one piece of advice we would give to companies seeking a manufacturing partner is to ensure the partner you choose has the size and scalability you require with a proven track record in quality of service.
Fenske: What are the forces driving medical device manufacturers to seek your technology/services over doing it in-house?
Higgins: Higher costs from ever-increasing regulatory standards coupled with global issues such as supply chain and labor shortages are major challenges for the market. Partnering with Integer can accelerate customers’ product development and increase speed to market, while access to our depth and breadth of technology furthers that competitive advantage.
Fenske: In what ways is your company able to aid in getting a product (project) to market faster?
Higgins: At Integer, our global project management organization (PMO) manages programs across our sites, leveraging our global quality system and intercompany supply of key components and technologies to accelerate development programs and achieve increased speed to market.
Integer is located at Medica/Compamed in Hall 8B, Booth/Stand P02.













