Sam Brusco, Associate Editor05.09.23
Everyone needs a periodic checkup, and that’s no different for the surgical robotics market. There’s been a great deal going on in the market over the past few months.
The ISU real-time intra-op surgical image analytics platform leverages augmented intelligence to reduce surgical variability and offers tools to reduce cognitive fatigue, while collecting data. Asensus will enable customer access portals and performance dashboards, and Google Cloud’s secure cloud data architecture will capture the data. Asensus will leverage Google Cloud’s ML tech to analyze the data and glean clinical intelligence that can be used by surgeons and hospitals, as well as continuously improve the ISU software for better intra-op clinical insight.
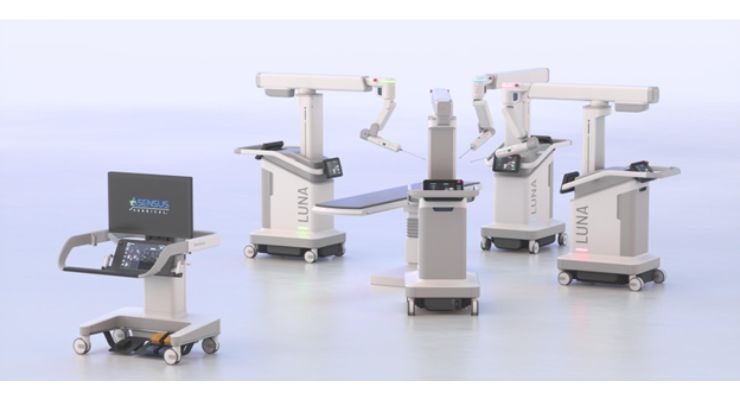
About a week later, Asensus revealed its next-generation surgical robot, LUNA. LUNA was designed based on feedback received from over 10,000 digital laparoscopic procedures. The next-gen system features advanced minimally invasive instrumentation, the first-ever digital interface between surgeon and console, and improved clinical intelligence tools.
Incremental features sets for Asensus’ ISU will include an analytical feature set for pre-op planning to let surgeons map out and plan for specific surgical actions intraoperatives using the ISU’s augmented intelligence. A new safety feature set, which includes “no-fly zone” functionality to identify and mark potential hazards during the operation, will help restrict instruments from moving into defined anatomical structures. A training and education set includes telestration so multiple team members can collaborate in real-time by annotating, highlighting, and drawing on a shared visual display of the surgical field.
“We have first-hand knowledge, shaped by thousands of real-world procedures and years of interactions with surgeons and hospitals, of what the market needs, which is much more than just a robot,” said Anthony Fernando, president and CEO of Asensus. “The best solution will materially reduce surgical variability and deliver improved outcomes. We are working to deliver that solution, a new era of surgery called Performance-Guided Surgery, the foundation of which will be our new LUNA Surgical System and the clinical intelligence capabilities provided to surgeons through the ongoing development of the ISU.”
Finally, in March the company’s flagship Senhance robot got FDA clearance for pediatric use. Senhance is already approved for pediatric patients in the EU and Japan.
The Galaxy System’s TiLT Technology features integrated tomosynthesis and augmented fluoroscopy. An always-on-camera bronchoscope enables direct visualization for the whole procedure, including at the time of biopsy. Four-way bronchoscope articulation helps navigate peripheral lesions. According to the company, Galaxy is the only robotic, navigated bronchoscopy system with a single-use, disposable bronchoscope intentionally designed to boost efficiency and workflow, as well as reduce risk of cross-contamination.
“While various technologies to diagnose lung cancer have been utilized over time, the diagnostic yield has remained relatively low,” said Jian Zhang, Ph.D., Noah Medical founder and CEO. “The Galaxy System is designed to close this gap in the market, giving clinicians a safe and easy-to-use platform to potentially improve diagnostic yield and produce better clinical outcomes. With this FDA clearance, we are excited to move into the commercial phase of our journey.”
In February, StitchKit gained FDA clearance for a new indication enabling insertion and removal through any existing 8 mm trocar incision while the surgeon watches the action on the laparoscope, allowing StitchKit use when no 12 mm trocars are in use. The technique involves removing one 8 mm trocar followed by direct placement of StitchKit into the surgical field through the trocar incision. At the end of the case, the surgeon removes StitchKit with the retrieval string, pulling it flush to robotic trocar and removing the trocar and StitchKit as one unit.
Asensus Surgical
First off, in February the company began a partnership with Google Cloud. The partnership will work to integrate Google Cloud’s secure cloud data architecture and machine learning (ML) tech to expand Asensus’ Performance-Guided Surgery (PGS) framework enabled via its Intelligent Surgical Unit (ISU). PGS may help surgeons perform more accurately and efficiently, help avoid complications, and improve outcomes.The ISU real-time intra-op surgical image analytics platform leverages augmented intelligence to reduce surgical variability and offers tools to reduce cognitive fatigue, while collecting data. Asensus will enable customer access portals and performance dashboards, and Google Cloud’s secure cloud data architecture will capture the data. Asensus will leverage Google Cloud’s ML tech to analyze the data and glean clinical intelligence that can be used by surgeons and hospitals, as well as continuously improve the ISU software for better intra-op clinical insight.
About a week later, Asensus revealed its next-generation surgical robot, LUNA. LUNA was designed based on feedback received from over 10,000 digital laparoscopic procedures. The next-gen system features advanced minimally invasive instrumentation, the first-ever digital interface between surgeon and console, and improved clinical intelligence tools.
Incremental features sets for Asensus’ ISU will include an analytical feature set for pre-op planning to let surgeons map out and plan for specific surgical actions intraoperatives using the ISU’s augmented intelligence. A new safety feature set, which includes “no-fly zone” functionality to identify and mark potential hazards during the operation, will help restrict instruments from moving into defined anatomical structures. A training and education set includes telestration so multiple team members can collaborate in real-time by annotating, highlighting, and drawing on a shared visual display of the surgical field.
“We have first-hand knowledge, shaped by thousands of real-world procedures and years of interactions with surgeons and hospitals, of what the market needs, which is much more than just a robot,” said Anthony Fernando, president and CEO of Asensus. “The best solution will materially reduce surgical variability and deliver improved outcomes. We are working to deliver that solution, a new era of surgery called Performance-Guided Surgery, the foundation of which will be our new LUNA Surgical System and the clinical intelligence capabilities provided to surgeons through the ongoing development of the ISU.”
Finally, in March the company’s flagship Senhance robot got FDA clearance for pediatric use. Senhance is already approved for pediatric patients in the EU and Japan.
Noah Surgical
Noah Surgical gained FDA clearance for its Galaxy system to provide bronchoscopic visualization of and access to patient airways in diagnostic and therapeutic procedures in March. The Galaxy System’s advanced imaging tech offers real-time location updates and is engineered to improve tool-in-lesion and diagnostic yield.The Galaxy System’s TiLT Technology features integrated tomosynthesis and augmented fluoroscopy. An always-on-camera bronchoscope enables direct visualization for the whole procedure, including at the time of biopsy. Four-way bronchoscope articulation helps navigate peripheral lesions. According to the company, Galaxy is the only robotic, navigated bronchoscopy system with a single-use, disposable bronchoscope intentionally designed to boost efficiency and workflow, as well as reduce risk of cross-contamination.
“While various technologies to diagnose lung cancer have been utilized over time, the diagnostic yield has remained relatively low,” said Jian Zhang, Ph.D., Noah Medical founder and CEO. “The Galaxy System is designed to close this gap in the market, giving clinicians a safe and easy-to-use platform to potentially improve diagnostic yield and produce better clinical outcomes. With this FDA clearance, we are excited to move into the commercial phase of our journey.”
Origami Surgical
Origami Surgical doesn’t produce a robotic system itself, but rather technologies to support surgical robotics. The company’s StitchKit portfolio enhances robotic-assisted surgery by letting the surgeon manage all aspects of suture use and needle disposal in clear view. It’s placed in the peritoneal cavity and used at the robotic surgical console. StitchKit has all the sutures required for the given robotic procedure, plus a SHARPS container where used needles are place throughout the case. After the surgery, StitchKit is removed from the surgical field with used needles stored safely inside. StitchKit, in summary, eliminates suture and needle passing by the bedside assistant to save time and eliminate needle loss inside the patient.In February, StitchKit gained FDA clearance for a new indication enabling insertion and removal through any existing 8 mm trocar incision while the surgeon watches the action on the laparoscope, allowing StitchKit use when no 12 mm trocars are in use. The technique involves removing one 8 mm trocar followed by direct placement of StitchKit into the surgical field through the trocar incision. At the end of the case, the surgeon removes StitchKit with the retrieval string, pulling it flush to robotic trocar and removing the trocar and StitchKit as one unit.