Rob Connelly, Engineering Manager, Trelleborg Healthcare & Medical11.10.21
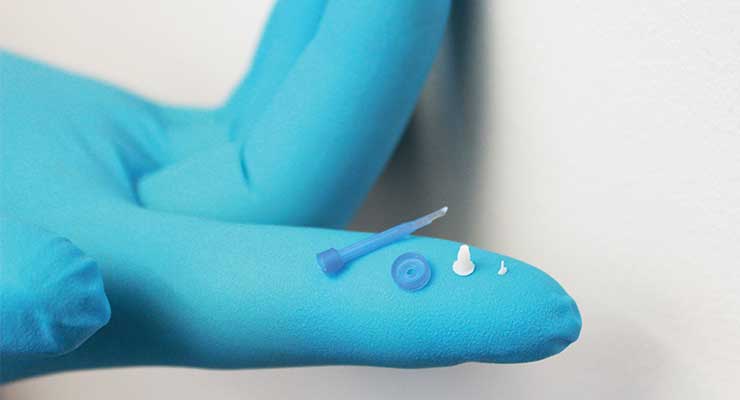
The demand for miniaturization of silicone and thermoplastic molded products for medical devices has heightened the importance of process validation, precision tool design, and skilled tool operators and engineers. According to Plastics News research, advancements and miniaturization in the medical device industry have improved healthcare around the globe, making treatments and monitoring of conditions simpler, less invasive, and more portable.
Innovations in the industry have also reduced contamination, made medical devices more affordable, and improved lives for patients of all ages. Results from a survey by Jabil Healthcare show miniaturization, flexible circuitry, and biometric sensors are leading to exciting new wearable devices that will help patients in recovery or with chronic issues. These devices collect and communicate data to healthcare providers and patients that can transform the way healthcare is managed.
One example of a miniaturized medical device is the Medtronic Micra AV, a cosmetically invisible device that makes surgery less invasive. The world’s smallest pacemaker with atrioventricular (AV) synchrony, it received FDA approval last year. Further miniaturization allowed fitment of the added function of AV block treatment to the existing Micra Transcatheter Pacing System (TPS); at a tenth of the size of a traditional pacemaker, it is equivalent in size to a large vitamin.1
Tools Rule, and Precision Is King
In order to miniaturize devices, its components must become smaller. This significantly increases the complexity of the manufacturing process and requires equipment, tooling, and processes that promote a high level of precision. Additionally, experts should design for manufacturability (DFM) to shorten time to market, improve future production yield, and ensure delivery of product in high volume.
Typical molding tools and processes are not usually capable of providing the quality and precision needed for miniature devices. Therefore, it’s important to partner with a supplier that understands the complexities of micromolding—a technology that presents new challenges with respect to mold precision and equipment control.
A robust micromolding process starts with high-quality injection molding tooling, which will determine the quality of the final product. All micromolding tools, whether producing a plastic or silicone component, require a high degree of precision in tolerances and shut-off surfaces to precisely control the flow of material into the mold cavity. To achieve this level of quality, high-precision machining equipment is needed for mold fabrication. Additionally, it’s important to have highly skilled toolmakers who understand the challenges of micromolding and have the experience to produce tooling with such small, high-precision features.
In micromolding processes, it is also vital to use equipment capable of injecting small doses of material into the mold cavity. Material doses or shot sizes of less than 1.0 g are typical for these micro-sized components. Shot-size control is critical for low viscosity liquid silicone rubber (LSR) and is, therefore, a major focus with micromolding equipment. Without tight control over shot size, 5 to 10 percent of micro part cavity volume can easily be “leaked” from the mold cavity. This produces excess material around the perimeter of the part, commonly referred to as flash.
For example, in a larger, more typical-sized molded component—the size of a hand, for instance—a small amount of flash is acceptable or may be removed in a secondary process. However, when a part is smaller than a pin head, the flash could be as large as the part itself, and flashless production becomes obligatory. This requires precision tooling, smaller injection units, better control of heat zones, and greater precision of injection units.
When working with a highly capable manufacturer of micromolded components on more advanced capabilities, such as multicomponent injection molding (also commonly referred to as 2K or 2-shot molding), two-component LSRs are ideal. Involving the simultaneous injection of LSR in combination with technical plastics, this highly advanced technique allows the combination of parts within an assembly into a single integrated component, eliminating potential assembly failures and dead space in which bacteria can breed. However, it requires sophisticated tool and process engineering.
A newer capability that is limited to only the most sophisticated micromolders is overmolding LSR or plastic over another substrate, such as metal or electronic components. This sophisticated technique can reduce the overall size of the assembled part and eliminate the need to assemble multiple small components together in a secondary process. Multicomponent and over molding can extend design options as no other technology can do, giving medical device developers the opportunity to go beyond function. As product profiles are virtually boundless, medical device designers have options they may not even be aware of being possible.
Validation, Please
In addition to high-precision molds and equipment, robust process validation and measurement methods are also critical to ensure miniaturized components are manufactured within specification. Validation of micromolded components is challenging due to process control complexity and difficulty in measuring, handling, and inspecting such small features. For example, micromolded parts are so small, static can literally cause the parts to fly away during handling. Therefore, special handling and static-mitigation devices are necessary throughout the manufacturing process.
Automated grippers are generally too large when demolding extremely small silicone parts, while ejectors are not suitable for silicone parts regardless of size. This is due to silicone’s propensity to flow into the clearance of an ejector system. Another alternative is using brushes to remove parts from the mold, but the tiny micromolded parts stick to the brush itself, rather than release from the mold and “fall” into a container below. The solution is specialized grippers designed specifically for micro-sized parts.
Packaging also needs to be considered in the production process, since standard polyethylene bags are not practical for extremely small parts. The parts will statically stick to the surface of the bag and be extremely difficult to remove. Therefore, parts may need to be packaged in small, hard plastic containers or on double-sided tape, for instance.
Gauge repeatability and reproducibility studies play an important role in ensuring the measurement processes for these micromolded components. These tests measure accuracy of the measurement process and help ensure measurements are repeatable by a single inspector and reproducible by multiple inspectors. As parts or part features and their associated tolerances become smaller, measurement equipment and methods increase in complexity. Methods and equipment must be engineered to achieve minimal measurement error and demonstrate statistical process capability and control.
Experienced micromolding manufacturers balance the trade-offs between increasing the number of cavities in a mold and the challenges of validating those additional cavities. Higher cavitation in a tool enables increased throughput and potentially lower costs. However, the additional cavitation increases the challenge of tooling and process control, which increases the complexity of validation. It’s also important to include in-process controls that enable components to be individually analyzed by cavity.
Extra Considerations for the Healthcare and Medical industry
For medical devices, cleanliness is paramount. Depending upon customer requirements, production can be in an “uncontrolled” or controlled environment of Class 100,000/ISO 8, or Class 10,000/ISO 7 cleanrooms.
Critical to the disciplines of any manufacturer involved in supplying “clean” product, whatever the size and whether from within or outside a classified cleanroom, is a Good Manufacturing Practice (GMP) discipline firmly rooted in the facility’s quality systems. Industry guidelines provide minimum requirements a manufacturer must meet to ensure products are of high quality and do not pose any risk to the consumer or public.
Experience Bolsters Success
The precise nature of micromolding demands consistency and a deep working knowledge of tooling, equipment, process and material science, and engineering. Experts are needed to understand the challenges and variations that exist in micromolding to anticipate and mitigate challenges before they occur. Finding a partner with longevity in the medical device industry and a depth of capabilities is invaluable for micromolding.
Conclusion
Micromolded parts are highly beneficial in the healthcare and medical industry because they facilitate development of medical devices to improve patients’ quality of life, shorten hospital stays, and reduce healthcare costs. The requirement for quality, less invasive surgical methods, and a larger demographic of aging people are driving demand for miniaturization. Device manufacturers should consider the critical elements of micromolding―tooling, equipment, process controls, knowledge of materials, measurement systems, process and product validation, and overall engineering expertise―and partner with experienced molders to ensure devices deliver the desired quality, consistency, and patient outcomes.
Reference
Rob Connelly is an engineering manager at Trelleborg Healthcare & Medical. He holds BS and MS degrees in industrial engineering from California Polytechnic State University, San Luis Obispo. Connelly has focused on developing solutions for molded silicone medical device components for over 16 years. Currently, he manages the Toolroom, New Product Development, and Automation Engineering teams at the Trelleborg Healthcare & Medical facility in Paso Robles, Calif.
Innovations in the industry have also reduced contamination, made medical devices more affordable, and improved lives for patients of all ages. Results from a survey by Jabil Healthcare show miniaturization, flexible circuitry, and biometric sensors are leading to exciting new wearable devices that will help patients in recovery or with chronic issues. These devices collect and communicate data to healthcare providers and patients that can transform the way healthcare is managed.
One example of a miniaturized medical device is the Medtronic Micra AV, a cosmetically invisible device that makes surgery less invasive. The world’s smallest pacemaker with atrioventricular (AV) synchrony, it received FDA approval last year. Further miniaturization allowed fitment of the added function of AV block treatment to the existing Micra Transcatheter Pacing System (TPS); at a tenth of the size of a traditional pacemaker, it is equivalent in size to a large vitamin.1
Tools Rule, and Precision Is King
In order to miniaturize devices, its components must become smaller. This significantly increases the complexity of the manufacturing process and requires equipment, tooling, and processes that promote a high level of precision. Additionally, experts should design for manufacturability (DFM) to shorten time to market, improve future production yield, and ensure delivery of product in high volume.
Typical molding tools and processes are not usually capable of providing the quality and precision needed for miniature devices. Therefore, it’s important to partner with a supplier that understands the complexities of micromolding—a technology that presents new challenges with respect to mold precision and equipment control.
A robust micromolding process starts with high-quality injection molding tooling, which will determine the quality of the final product. All micromolding tools, whether producing a plastic or silicone component, require a high degree of precision in tolerances and shut-off surfaces to precisely control the flow of material into the mold cavity. To achieve this level of quality, high-precision machining equipment is needed for mold fabrication. Additionally, it’s important to have highly skilled toolmakers who understand the challenges of micromolding and have the experience to produce tooling with such small, high-precision features.
In micromolding processes, it is also vital to use equipment capable of injecting small doses of material into the mold cavity. Material doses or shot sizes of less than 1.0 g are typical for these micro-sized components. Shot-size control is critical for low viscosity liquid silicone rubber (LSR) and is, therefore, a major focus with micromolding equipment. Without tight control over shot size, 5 to 10 percent of micro part cavity volume can easily be “leaked” from the mold cavity. This produces excess material around the perimeter of the part, commonly referred to as flash.
For example, in a larger, more typical-sized molded component—the size of a hand, for instance—a small amount of flash is acceptable or may be removed in a secondary process. However, when a part is smaller than a pin head, the flash could be as large as the part itself, and flashless production becomes obligatory. This requires precision tooling, smaller injection units, better control of heat zones, and greater precision of injection units.
When working with a highly capable manufacturer of micromolded components on more advanced capabilities, such as multicomponent injection molding (also commonly referred to as 2K or 2-shot molding), two-component LSRs are ideal. Involving the simultaneous injection of LSR in combination with technical plastics, this highly advanced technique allows the combination of parts within an assembly into a single integrated component, eliminating potential assembly failures and dead space in which bacteria can breed. However, it requires sophisticated tool and process engineering.
A newer capability that is limited to only the most sophisticated micromolders is overmolding LSR or plastic over another substrate, such as metal or electronic components. This sophisticated technique can reduce the overall size of the assembled part and eliminate the need to assemble multiple small components together in a secondary process. Multicomponent and over molding can extend design options as no other technology can do, giving medical device developers the opportunity to go beyond function. As product profiles are virtually boundless, medical device designers have options they may not even be aware of being possible.
Validation, Please
In addition to high-precision molds and equipment, robust process validation and measurement methods are also critical to ensure miniaturized components are manufactured within specification. Validation of micromolded components is challenging due to process control complexity and difficulty in measuring, handling, and inspecting such small features. For example, micromolded parts are so small, static can literally cause the parts to fly away during handling. Therefore, special handling and static-mitigation devices are necessary throughout the manufacturing process.
Automated grippers are generally too large when demolding extremely small silicone parts, while ejectors are not suitable for silicone parts regardless of size. This is due to silicone’s propensity to flow into the clearance of an ejector system. Another alternative is using brushes to remove parts from the mold, but the tiny micromolded parts stick to the brush itself, rather than release from the mold and “fall” into a container below. The solution is specialized grippers designed specifically for micro-sized parts.
Packaging also needs to be considered in the production process, since standard polyethylene bags are not practical for extremely small parts. The parts will statically stick to the surface of the bag and be extremely difficult to remove. Therefore, parts may need to be packaged in small, hard plastic containers or on double-sided tape, for instance.
Gauge repeatability and reproducibility studies play an important role in ensuring the measurement processes for these micromolded components. These tests measure accuracy of the measurement process and help ensure measurements are repeatable by a single inspector and reproducible by multiple inspectors. As parts or part features and their associated tolerances become smaller, measurement equipment and methods increase in complexity. Methods and equipment must be engineered to achieve minimal measurement error and demonstrate statistical process capability and control.
Experienced micromolding manufacturers balance the trade-offs between increasing the number of cavities in a mold and the challenges of validating those additional cavities. Higher cavitation in a tool enables increased throughput and potentially lower costs. However, the additional cavitation increases the challenge of tooling and process control, which increases the complexity of validation. It’s also important to include in-process controls that enable components to be individually analyzed by cavity.
Extra Considerations for the Healthcare and Medical industry
For medical devices, cleanliness is paramount. Depending upon customer requirements, production can be in an “uncontrolled” or controlled environment of Class 100,000/ISO 8, or Class 10,000/ISO 7 cleanrooms.
Critical to the disciplines of any manufacturer involved in supplying “clean” product, whatever the size and whether from within or outside a classified cleanroom, is a Good Manufacturing Practice (GMP) discipline firmly rooted in the facility’s quality systems. Industry guidelines provide minimum requirements a manufacturer must meet to ensure products are of high quality and do not pose any risk to the consumer or public.
Experience Bolsters Success
The precise nature of micromolding demands consistency and a deep working knowledge of tooling, equipment, process and material science, and engineering. Experts are needed to understand the challenges and variations that exist in micromolding to anticipate and mitigate challenges before they occur. Finding a partner with longevity in the medical device industry and a depth of capabilities is invaluable for micromolding.
Conclusion
Micromolded parts are highly beneficial in the healthcare and medical industry because they facilitate development of medical devices to improve patients’ quality of life, shorten hospital stays, and reduce healthcare costs. The requirement for quality, less invasive surgical methods, and a larger demographic of aging people are driving demand for miniaturization. Device manufacturers should consider the critical elements of micromolding―tooling, equipment, process controls, knowledge of materials, measurement systems, process and product validation, and overall engineering expertise―and partner with experienced molders to ensure devices deliver the desired quality, consistency, and patient outcomes.
Reference
Rob Connelly is an engineering manager at Trelleborg Healthcare & Medical. He holds BS and MS degrees in industrial engineering from California Polytechnic State University, San Luis Obispo. Connelly has focused on developing solutions for molded silicone medical device components for over 16 years. Currently, he manages the Toolroom, New Product Development, and Automation Engineering teams at the Trelleborg Healthcare & Medical facility in Paso Robles, Calif.