Ryan Oleksy, Co-Founder, Next Life Medical Corporation; Advisor, Sauron Group03.04.20
It’s 6:50 a.m., and the day starts with the usual dose of coffee and news. So far, it seems like any other typical Tuesday—until 7 a.m., that is, when the news anchor recaps the morning’s top global stories. The headlines, of course, are a doom and gloom amalgamation of politics, ethical lapses, terror, sadness, and war. But today, the headlines hit closer to home. There’s a report of an earthquake in a Far Eastern country—the area home to a key supplier you placed important purchase orders with just two days prior.
After processing the unfolding disaster for all those impacted, you naturally begin to play the infamous game Twenty Questions in your head. This time, though, the game is not for fun but for real: How many suppliers are in the region? Are they impacted? Are their employees OK? How about their families? What’s the damage? Are the production tools OK? Will supply be impacted? What should be done next? How can they be contacted? For seasoned supply chain experts, it doesn’t take long to mentally process 20 questions at warp speed—or more accurately—panic speed.
Whether it’s an earthquake in the Far East or the uncontrollable spread of the coronavirus, these incidents highlight the reality and complexity associated with leveraging and managing a global supply chain. They also accentuate the importance of always being prepared for the unexpected.
But there is good news. Several years ago, MPO published a column on implementing a more stringent supply chain risk monitoring (SCRM) program for medtech organizations. Fortunately, this program does not require esoteric concepts that demand a formal education in their philosophy or execution. However, the importance lies in having the right data about supply chains or chain of chains from top to bottom, left to right, and being able to take that data and convert it to actionable intelligence when mayhem strikes.
Risk monitoring helps companies prevent or limit the negative impact of supply chain issues on both themselves and their customers. But have companies addressed this important topic or have they simply “checked the box”? Do organizations truly look further than tier 1 suppliers or do they assume they “have it covered with great suppliers”? As companies experience growth, their supply chains typically grow larger and more complex. The global supplier network required to feed materials, components. and services to medical product lines in order to fuel revenue and profit growth also fuel increased complexity and risk. And, more times than not, risk monitoring becomes a low priority or is done minimally to simply “check the box” and satisfy concerns from a company’s legal department or insurance provider (or both).
A holistic, proactive, multi-tiered SCRM program is critical to help avoid, mitigate, and manage profit-killing disruptions to the supply chain or the loss of human lives. Sole-sourced therapy can experience a supply disruption due to any number of incidents that can take place.
There is no cookie-cutter approach to SCRM because organizations and their supply chains differ dramatically. Some are domestic, some are global; some have redundant capabilities, some do not; some have life-sustaining products, some do not. It’s important for companies to define a vision for what risk means to the organization and then put together a program to monitor, mitigate, and manage that risk.
One approach to effectively building a SCRM is to create a hypothetical incident that wreaks havoc on suppliers. During a disruptive incident, teams must be able to address various concerns; thus, 20 questions might include such queries as:
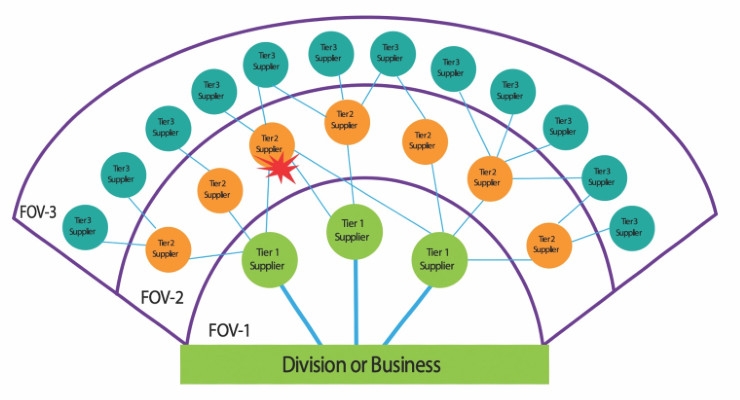
During a critical incident, it can take days or weeks for companies to understand and analyze the impact on a tier 1 supplier, and even longer for tier 2 or 3 suppliers. This lack of supply chain visibility is a “Field of Vision”(FOV) problem that plagues supply chains of all sizes and complexities.
In complex, multi-level supply chains, the FOV problem is exacerbated because a tier 2 vendor provides product for multiple tier 1 suppliers (see diagram). This is known as implosion or imploded leverage risk. Increased reliance on tier 2 players can be devastating for multiple supply chains and companies, as the latter seldom has knowledge of providers existing beyond the tier 1 realm. Consider, for example, an earthquake that destroys a single sourced tier 2 supplier of critical components to three tier 1 players. With limited supply chain FOV, in the absence of a SCRM program, a company may not be aware of the impending disruption until it is notified directly by the tier 1 supplier.
Think about the impact of requalification/validation of a limited biologic supply from a tier 2 provider imploding upward into multiple tier 1 products. The imploded cost to the business can be unfathomable at times. Not only is it costly, it can be incredibly time consuming (depending on the material) and potentially deadly to patients dependent on life-sustaining products.
Increasing FOV depth makes it easier to triage adverse events, run simulation models, and plan for known possible impacts. As mentioned earlier, companies would be well-served to develop a list of incidents that can occur and their potential impacts. In the case of hurricanes and monsoons, alerts that notify supply chain management personnel of these storms’ potential impact can allow organizations to possibly position inventory ahead of landfall and avoid a disruption in supply, or, at the very least, mitigate some of the overall effect.
It is a known fact that logistics and freight vendors are impacted by these kinds of storms. It is not uncommon for wide service areas to be affected well in advance of a potential impact from a hurricane. In the past year, hurricanes have impacted the delivery capabilities of UPS, Fedex, the U.S. Postal Service, and several other carriers. This caused not only a direct impact in those organizations’ ability to obtain materials, but also impeded fulfillment of end product to customers. It makes more sense to plan ahead by shipping some product ahead of time, rather than dealing with expedited rates or backlog on the backend of these storms. And even though it is often viewed as heresy to finance teams, inventory can be a savior if positioned correctly.
It is also very important that increased FOV apply to geopolitical trade risks and potential human rights violations as well. These issues can severely impact the cost of conducting business with various suppliers. In the age of tariffs being the conventional weapon of choice during trade war skirmishes, supply chain managers are often caught scrambling in the trenches to identify the real financial impact to the business. There is also mounting evidence that human rights violations are having a direct impact on medtech supply chains. A perfect example of this is the closure of key mineral extraction sites, mining copper and cobalt in the DRC (Democratic Republic of the Congo).
Cobalt is a key component in the manufacturing process for lithium batteries, and as of November 2019, the largest mine (producing 20 percent of global cobalt supply) shut down due to several human rights violation allegations, lack of access to sulphuric acid, and profitability issues. Companies with a SCRM program in place were monitoring this commodity and saw warning signs months ahead of the closure and had the opportunity to negotiate prioritized supply with their battery vendor, or at the very least, attempted to lock in fair pricing.
The purpose of this article is not to scare medtech firms into a doomsday prepping frenzy, but rather trigger critical thought about their current programs. Companies should determine whether they have an SCRM program in place that adequately monitors, mitigates, and manages mayhem. Such a program allows for triaging a disruption anywhere in the supply chain at any moment, even in tiers 2 or 3.
Companies that lack an SCRM program might want to use the coronavirus as inspiration and implement one quickly. There are several firms with the specialized knowledge required to ensure device manufacturers are prepared for the worst while hoping for the best. Bottom line: When it’s truly time to play Twenty Questions, companies should have the answers to those queries.
Ryan Oleksy is co-founder of Next Life Medical and an advisor at Sauron Group. He can be reached at ryan@nextlifemedical.com or roleksy@saurongroup.com.
After processing the unfolding disaster for all those impacted, you naturally begin to play the infamous game Twenty Questions in your head. This time, though, the game is not for fun but for real: How many suppliers are in the region? Are they impacted? Are their employees OK? How about their families? What’s the damage? Are the production tools OK? Will supply be impacted? What should be done next? How can they be contacted? For seasoned supply chain experts, it doesn’t take long to mentally process 20 questions at warp speed—or more accurately—panic speed.
Whether it’s an earthquake in the Far East or the uncontrollable spread of the coronavirus, these incidents highlight the reality and complexity associated with leveraging and managing a global supply chain. They also accentuate the importance of always being prepared for the unexpected.
But there is good news. Several years ago, MPO published a column on implementing a more stringent supply chain risk monitoring (SCRM) program for medtech organizations. Fortunately, this program does not require esoteric concepts that demand a formal education in their philosophy or execution. However, the importance lies in having the right data about supply chains or chain of chains from top to bottom, left to right, and being able to take that data and convert it to actionable intelligence when mayhem strikes.
Risk monitoring helps companies prevent or limit the negative impact of supply chain issues on both themselves and their customers. But have companies addressed this important topic or have they simply “checked the box”? Do organizations truly look further than tier 1 suppliers or do they assume they “have it covered with great suppliers”? As companies experience growth, their supply chains typically grow larger and more complex. The global supplier network required to feed materials, components. and services to medical product lines in order to fuel revenue and profit growth also fuel increased complexity and risk. And, more times than not, risk monitoring becomes a low priority or is done minimally to simply “check the box” and satisfy concerns from a company’s legal department or insurance provider (or both).
A holistic, proactive, multi-tiered SCRM program is critical to help avoid, mitigate, and manage profit-killing disruptions to the supply chain or the loss of human lives. Sole-sourced therapy can experience a supply disruption due to any number of incidents that can take place.
There is no cookie-cutter approach to SCRM because organizations and their supply chains differ dramatically. Some are domestic, some are global; some have redundant capabilities, some do not; some have life-sustaining products, some do not. It’s important for companies to define a vision for what risk means to the organization and then put together a program to monitor, mitigate, and manage that risk.
One approach to effectively building a SCRM is to create a hypothetical incident that wreaks havoc on suppliers. During a disruptive incident, teams must be able to address various concerns; thus, 20 questions might include such queries as:
- What plants are at risk?
- What tier 1, 2, 3...suppliers are impacted or potentially impacted in tertiary events?
- What product lines and part numbers are impacted?
- Who are the key supplier contacts?
- How can these suppliers be contacted? What technology can be used?
- What is the likely financial impact to the business?
- How should the disruption be mitigated to minimize impact?
During a critical incident, it can take days or weeks for companies to understand and analyze the impact on a tier 1 supplier, and even longer for tier 2 or 3 suppliers. This lack of supply chain visibility is a “Field of Vision”(FOV) problem that plagues supply chains of all sizes and complexities.
In complex, multi-level supply chains, the FOV problem is exacerbated because a tier 2 vendor provides product for multiple tier 1 suppliers (see diagram). This is known as implosion or imploded leverage risk. Increased reliance on tier 2 players can be devastating for multiple supply chains and companies, as the latter seldom has knowledge of providers existing beyond the tier 1 realm. Consider, for example, an earthquake that destroys a single sourced tier 2 supplier of critical components to three tier 1 players. With limited supply chain FOV, in the absence of a SCRM program, a company may not be aware of the impending disruption until it is notified directly by the tier 1 supplier.
Think about the impact of requalification/validation of a limited biologic supply from a tier 2 provider imploding upward into multiple tier 1 products. The imploded cost to the business can be unfathomable at times. Not only is it costly, it can be incredibly time consuming (depending on the material) and potentially deadly to patients dependent on life-sustaining products.
Increasing FOV depth makes it easier to triage adverse events, run simulation models, and plan for known possible impacts. As mentioned earlier, companies would be well-served to develop a list of incidents that can occur and their potential impacts. In the case of hurricanes and monsoons, alerts that notify supply chain management personnel of these storms’ potential impact can allow organizations to possibly position inventory ahead of landfall and avoid a disruption in supply, or, at the very least, mitigate some of the overall effect.
It is a known fact that logistics and freight vendors are impacted by these kinds of storms. It is not uncommon for wide service areas to be affected well in advance of a potential impact from a hurricane. In the past year, hurricanes have impacted the delivery capabilities of UPS, Fedex, the U.S. Postal Service, and several other carriers. This caused not only a direct impact in those organizations’ ability to obtain materials, but also impeded fulfillment of end product to customers. It makes more sense to plan ahead by shipping some product ahead of time, rather than dealing with expedited rates or backlog on the backend of these storms. And even though it is often viewed as heresy to finance teams, inventory can be a savior if positioned correctly.
It is also very important that increased FOV apply to geopolitical trade risks and potential human rights violations as well. These issues can severely impact the cost of conducting business with various suppliers. In the age of tariffs being the conventional weapon of choice during trade war skirmishes, supply chain managers are often caught scrambling in the trenches to identify the real financial impact to the business. There is also mounting evidence that human rights violations are having a direct impact on medtech supply chains. A perfect example of this is the closure of key mineral extraction sites, mining copper and cobalt in the DRC (Democratic Republic of the Congo).
Cobalt is a key component in the manufacturing process for lithium batteries, and as of November 2019, the largest mine (producing 20 percent of global cobalt supply) shut down due to several human rights violation allegations, lack of access to sulphuric acid, and profitability issues. Companies with a SCRM program in place were monitoring this commodity and saw warning signs months ahead of the closure and had the opportunity to negotiate prioritized supply with their battery vendor, or at the very least, attempted to lock in fair pricing.
The purpose of this article is not to scare medtech firms into a doomsday prepping frenzy, but rather trigger critical thought about their current programs. Companies should determine whether they have an SCRM program in place that adequately monitors, mitigates, and manages mayhem. Such a program allows for triaging a disruption anywhere in the supply chain at any moment, even in tiers 2 or 3.
Companies that lack an SCRM program might want to use the coronavirus as inspiration and implement one quickly. There are several firms with the specialized knowledge required to ensure device manufacturers are prepared for the worst while hoping for the best. Bottom line: When it’s truly time to play Twenty Questions, companies should have the answers to those queries.
Ryan Oleksy is co-founder of Next Life Medical and an advisor at Sauron Group. He can be reached at ryan@nextlifemedical.com or roleksy@saurongroup.com.