Sam Brusco, Associate Editor09.09.19
Alteration of nerve activity through targeted electrical stimulation has been practiced for millennia. There are records as early as 46 A.D. of electrical torpedo fish—or electric rays—being applied directly to a painful area to induce relief. The resulting shock briefly stuns the nervous system, provoking immediate and residual numbness. As such, electric rays became the first transcutaneous electrical nerve stimulation (TENS) devices and were often used to treat gouty arthritis.
The practice has understandably since evolved, and researchers worldwide are investigating altering nerve activity in specific nerves or neurological sites in the body. Applying small, electrical stimuli to specific nerves has been found to have enormous potential in treating various conditions from epilepsy to mental health to heart disease. This method of treating chronic conditions is known as neuromodulation or neurostimulation (neurostim). Eventually, neurostim devices were implanted in the body at specific sites for more direct application of electrical pulses.
“Although originally invasive in nature, neurostimulation devices have evolved from invasive procedures to external electrical stimulators, such as vagus nerve stimulation (VNS) devices to transcutaneous vagus nerve stimulation (TVNS) devices,” said David Novotny, general manager and global head of medical device and diagnostic research for ICON plc, a Dublin, Ireland-based global provider of outsourced development and commercialization services to pharmaceutical, biotechnology, medical device, and government and public health organizations. “Moreover, engineers have focused on reducing battery size in implantable devices, making implantable stimulators a more attractive option for patients concerned with cosmetic appearances.”
The core neurostimulation therapies of spinal cord stimulation, deep brain stimulation, and vagus nerve stimulation have been continuously advancing. For example, spinal cord stimulation was initially centered on hardware innovation and the focus has shifted to waveform innovations, strategies that stimulate the spinal cord by a different mechanism of action like high-frequency or burst stimulation, which consists of closely packed high-frequency impulses, followed by a quiescent period. Other advancements include systems that automatically adjust treatment and shrinking the devices for the patient’s benefit.
“The introduction of more advanced waveforms and stimulation frequencies offers greater programming flexibility to meet patients’ needs,” commented Brian LaPrade, product management director, cardiac rhythm management & neuromodulation at Integer Holdings Corp., a Plano, Texas-based medical device outsource manufacturer. “We also see the development of closed-loop systems that enable devices to automatically adjust based on ‘detected’ responses, miniaturization of devices to improve patient comfort and reduce procedure invasiveness (including use of externally powered ‘injectable’ devices), and a shift to Bluetooth communication between the implanted device and externals.”
The neurostim market has flourished as proof of these devices’ effectiveness has grown. Neurostim’s scientific and technological advancement continues due to the increase in the geriatric population and the rise of the devices’ safety and efficacy. Pamela Kennedy, R.N.—ICON plc’s executive director, business development, medical device and diagnostic research—outlined the myriad external and internal neurostim technologies and how they are impacting various therapeutic areas:
External neurostim devices:
“Neurostimulation technology offers potential to improve upon traditional treatment options in the areas of epilepsy, Parkinson’s, depression, sleep apnea, dystonia (spasticity), pain management, obesity/gastric, migraine, and heart disease,” Kennedy explained. “Also, neurostimulation devices are improving upon traditional treatments for these conditions by offering an affordable and non-invasive treatment option in a financially overburdened healthcare system. Moreover, in the case of Parkinson’s disease, epilepsy, and urinary incontinence conditions, neurostimulation offers hope for patients that previously had little to no treatment options and poor quality of life.”
First used to treat pain in 1967, SCS delivers mild electrical stimulation to the nerves along the spinal column, modifying nerve activity to minimize pain signals to the brain. The therapy began routine use in the 1980s, and the FDA first approved SCS in 1989 to relieve chronic pain from nerve damage in the trunk, arms, or legs. According to the International Neuromodulation Society, SCS therapy accounts for about 70 percent of all neuromodulation treatments, and about 34,000 patients undergo SCS implants each year worldwide.
“While vagus nerve stimulation and deep brain stimulation led the industry in the mid-1990s, spinal cord stimulation continues to hold the largest market share today,” Kennedy noted.
SCS treats pain that is neuropathic in origin—meaning it arises from nerve damage and doesn’t serve a protective purpose. The damage usually occurs as a result of accident, injury, or disease and SCS therapy is most commonly indicated for the treatment of neuropathic back and leg pain. Since the 80s, SCS therapy has advanced in ways that tailor the treatment to a patient’s individual needs. Newer lead designs allow more precise control of the electrical field, and increasingly sophisticated devices offer a myriad of stimulation parameters. Some SCS devices can even achieve pain reduction without evoking perceptible sensations.
Medical device makers featuring a cardiac rhythm management portfolio—Medtronic, Abbott Laboratories, and Boston Scientific, for example—typically also offer SCS and other neurostim devices because both devices’ mechanisms of action similarly involve the delivery of electrical pulses to a specific site via a lead. The latest SCS technologies incorporate peripheral tools to assist in recording/analyzing the treatment and/or target novel neurological areas to enhance therapy. Medtronic claims its Intellis SCS platform with AdaptiveStim is the smallest fully implantable spinal cord neurostimulator and includes a clinician programmer and reporting via tablet to track a patient’s progress. Abbott’s Proclaim DRG targets the dorsal root ganglion (DRG) to stimulate primary sensory neurons, a processing point of sensory information. Boston Scientific claims its Spectra WaveWriter SCS system is the first designed to personalize pain relief through combination therapy and the simplicity of waveform automation.
Rather than blocking signals to the brain, DBS involves implanting electrodes within certain areas of the brain. These electrodes produce electrical impulses that regulate abnormal impulses or affect specific cells and chemicals within the brain. The amount of DBS is controlled by a pacemaker-like device placed under the skin in the upper chest, and a wire traveling under the skin connects it to the electrodes in the brain.
DBS is currently approved by the FDA to treat dystonia (a movement disorder), epilepsy, essential tremor, obsessive-compulsive disorder, and Parkinson’s disease. It is also undergoing evaluation as a potential treatment for a variety of conditions: addiction, chronic pain, cluster headache, dementia, major depressive disorder, Huntington’s disease, multiple sclerosis, stroke recovery, Tourette syndrome, and traumatic brain injury. As the procedure to install a DBS system involves both brain surgery and chest wall surgery, the treatment is reserved for people who aren’t able to control their symptoms with medications.
Modern DBS systems also feature enhancements that improve the clinician and patient interaction with the device. Medtronic’s Activa DBS portfolio contains the first rechargeable device and has 15-year longevity. Abbott’s Infinity DBS system is controlled with an Apple iPad mini mobile digital device so the doctor can adjust the setting for personalized, discreet therapy management. Boston Scientific claims its Vercise DBS system was the first engineered for precise neural targeting to customize therapy for patients with Parkinson’s disease, primary and secondary dystonia, and essential tremor. Its Vercise Gevia DBS system was approved for MRI labeling toward the end of August.
New therapeutic areas have been uncovered as research in neurostim continues. For example, continuous positive airway pressure (CPAP) is the usual treatment option for obstructive sleep apnea (OSA). Many people can’t tolerate the cumbersome nightly therapy. The FDA approved Inspire Upper Airway Stimulation in 2014 to treat OSA in those that can’t tolerate CPAP, and it has found success. Further, VNS, which has historically been used to treat epilepsy, has found success in curbing obesity as well.
“Neurostimulation has been leveraged to treat sleep apnea by stimulating the activity of the hypoglossal nerve, which supports the activity of the tongue,” said Novotny. “In sleep apnea patients, because the tongue relaxes and collapses into the upper airway during sleep, the device stimulates the tongue to help prevent obstructions in the upper airway. Neurostimulation has also been shown to help treat obesity by stimulating the vagus nerve, which serves as the signaling connection between the stomach and the brain.”
Neurostim technology has the potential to be an aid in stemming opioid abuse, as well. The symptoms of opioid withdrawal can be incredibly distressing, come on quickly, and are one of the main reasons patients with physical dependence on the drugs have so much trouble stopping them. Early symptoms usually begin in the first 24 hours depending on the intensity of the patient’s physical dependence on the drug and include anxiety, cramping, muscle aches, restlessness, sweating, tears, and a runny nose. Later, more intense symptoms like diarrhea, abdominal cramping, nausea and vomiting, rapid heartbeat, and hypertension set in after the first day.
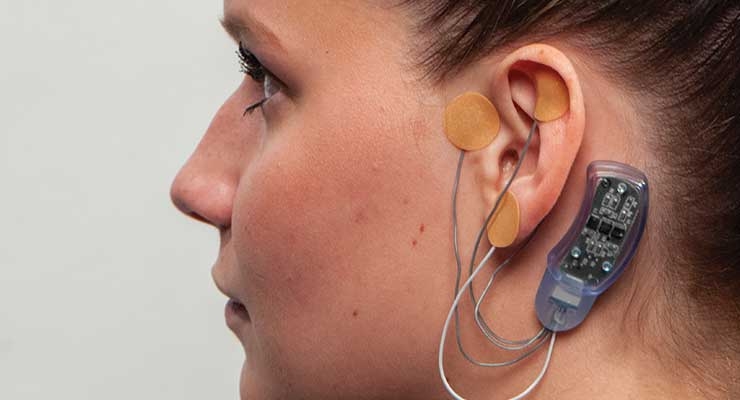
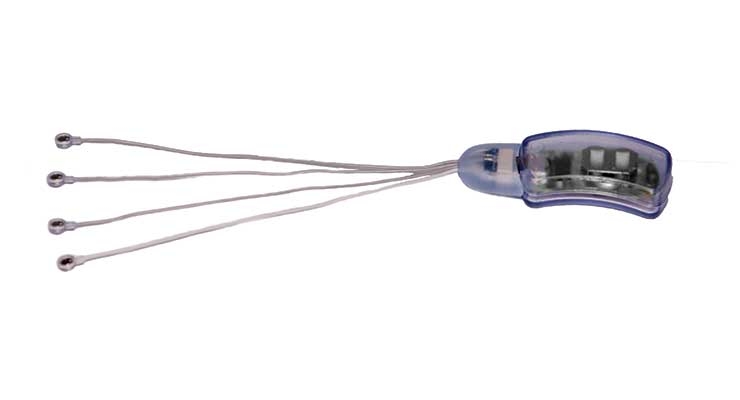
Innovative Health Solutions’ (IHS) NSS-2 BRIDGE is a percutaneous nerve field stimulator device system that can be used as an aid to reduce symptoms of opioid withdrawal through application to branches of specific cranial nerves, as well as branches of the occipital nerves identified via transillumination. NSS-2 BRIDGE received FDA clearance in November 2017 and has since been used by doctors and treatment centers at the local and state level to assist thousands of people suffering from opioid withdrawal symptoms.
“The NSS-2 BRIDGE goes behind the patient’s ear and includes micro-needle arrays that percutaneously implant in and around the ear and send gentle electrical impulses to branches of the nerves that lead to central brain regions involved in symptoms of withdrawal, particularly the amygdala and spinal cord,” explained an IHS spokesperson. “Research study results show an 84.6 percent reduction of withdrawal symptoms in as little as 60 minutes.”
The device helps to alleviate the opioid withdrawal symptoms of nausea, diarrhea, vomiting, abdominal pain, anxiety, and restlessness. Relieving these symptoms in as little as 60 minutes can go a long way to aid in a long-term recovery plan from opioid withdrawal.
“Greater early decreases in withdrawal scores have been shown to significantly correlate with long-term treatment success,” the IHS spokesperson went on.
IHS also gained FDA clearance in June for the first treatment indicated to reduce pain in children dealing with functional abdominal pain associated with irritable bowel syndrome (IBS). “There are currently no other approved therapies for IBS in children. Currently approved medications for IBS in adults all work at the level of the gut and are often used off-label in children, despite the risk of serious side effects,” the company spokesperson explained. “Also, the intensity of withdrawal symptoms has been correlated with treatment drop-out. Therefore, treatment with NSS-2 BRIDGE is likely to enhance long-term recovery, regardless of the type of medication-assisted therapy used. It allows patients to overcome a major hurdle and is meant to enhance long-term recovery.”
IB-Stim targets brain regions involved in pain processing, unlike traditional therapies for IBS that target the gut. IB-Stim is also nonsurgical, targeting central brain areas through stimulation of cranial nerves in the ear. Prior to FDA clearance, the device underwent a double-blind, randomized, controlled trial that included 104 patients aged 11-18 with functional abdominal pain disorders, measuring the effects of IB-Stim compared to placebo on several pain aspects including worst pain and the pain-frequency-severity-duration composite score. It also measured patients’ overall symptom improvement based on the Symptom Response Scale. The company’s sub-analysis of IBS subjects showed before IB-Stim treatment, 70 percent of patients failed to improve with four medications trialed. However, patients treated with IB-Stim had an 81 percent improvement in global symptoms with no serious adverse events, and minimal to no side effects.
“IB-Stim treats functional abdominal pain associated with IBS in patients ages 11-18,” the company spokesperson commented. “The IB-Stim is a non-surgical device that is placed behind the patient’s ear during an outpatient visit and works by sending gentle electrical impulses into cranial nerve bundles located in the ear. This stimulation targets brain areas involved in processing pain and aids in the reduction of functional abdominal pain associated with IBS.”
Bioelectronic medicine merges molecular medicine, neuroscience, engineering, and computing to develop devices to diagnose and treat diseases, and represents a potential revolution in disease management. The discipline arose from discoveries of mechanisms for neural control of biological processes that underlie disease, and development of technologies to therapeutically modulate those specific neural circuits using electrons in place of drugs. A bioelectronic medical device uses precise electrical pulses like a neurostim technology, but the pulses are used to activate systemic responses for the treatment of certain chronic diseases.
“Bioelectronic medicine differs from traditional neuromodulation therapies by addressing the underlying pathophysiology to provide a potential cure for the disease versus simply masking the symptoms of disease,” explained Ankit Shah, senior director of commercialization and marketing for SetPoint Medical, a Valencia, Calif.-based bioelectronic medicine company that is dedicated to treating patients with chronic autoimmune diseases.
SetPoint Medical’s unique approach to bioelectronic medicine aims to return the body to its natural immunological “set point” by using vagus nerve stimulation to restore immunological homeostasis. It does so through targeted electrical pulses to the vagus nerve to trigger the inflammatory reflex, which regulates and restores balance to the immune system. The vagus nerve also innervates the spleen, which is also responsible for responding to infection and inflammation. Activating the inflammatory reflex alters the phenotype of inflammatory cells in the spleen, reducing the amount of circulating cytokines and dampening systemic inflammation.
Current vagus nerve stimulating devices require two incisions to implant a large pulse generator in the chest with a wired lead tunneled to the neck. SetPoint’s device, however, is a miniaturized, rechargeable system with an integrated electrode and no lead, so it can be implanted with a single incision, minimally invasive procedure on the nerve. This reduction in the implant’s footprint substantially benefits patients, delivering a proprietary pulse pattern for autoimmune disease treatment.
“We have conducted multiple clinical studies to better understand the safety and effectiveness of bioelectronic medicine as a potential alternative to treatment of chronic, inflammation-mediated autoimmune diseases,” said Shah. “We are initially focused on the development of our device for the treatment of rheumatoid arthritis (RA) as a lead indication. Our device also has promising data for Crohn’s disease as well as multiple sclerosis (MS) in various stages of development.”
RA is a challenging condition to treat because according to SetPoint, 30-40 percent of RA patients don’t adequately respond to or are intolerant of biologic therapies—those therapies already have potentially serious side effects, and patient compliance is a serious issue. These biologic therapies also financially burden the healthcare system because some of the highest revenue grossing drugs are indicated for patients with RA and other autoimmune diseases.
“There is a major unmet need for novel treatment options for autoimmune diseases that are more efficacious, less immunosuppressive, and more cost-effective than currently approved therapies,” noted Shah.
The company recently posted positive results from its U.S. pilot IDE study evaluating its bioelectronic medicine device for RA treatment. SetPoint Medical’s bioelectronic medicine was found to be well-tolerated with no device-related adverse events through 12 weeks. Five of the 10 active stimulation patients who had failed multiple biologic and targeted synthetic therapies had clinically meaningful improvement in RA signs and symptoms as measured by Disease Activity Score (DAS28-CRP) and Clinical Disease Activity Index (DCAI), two validated disease measurement instruments. Two of those patients even achieved DAS28-CRP remission.
“Unlike traditional biologic therapies which aim to eliminate the bioavailability of a single pro-inflammatory cytokine, SetPoint’s bioelectronic medicine approach has been shown to suppress a range of inflammatory cytokines by 30-70 percent without eliminating the bioavailability of any single cytokine,” said Shah. “In doing so, inflammation can be reduced without causing significant immunosuppression, which is a major risk factor for serious side effects like systemic infections associated with almost all currently available therapies for RA.”
In addition to clinical studies in RA and Crohn’s disease, SetPoint is currently studying the potential of its bioelectronic medicine for MS. Preclinical research is ongoing, and according to the company, results show promising early signals of efficacy in animal MS models.
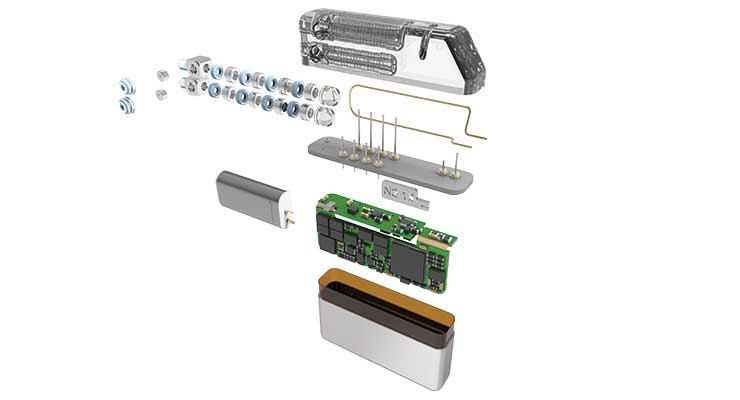
Neurostim OEMs, like any other medical device maker, aim to bring their innovative therapies to market as fast and as cost-effectively as possible. When this challenge involves more capacity and cost than the device maker has, they might consider turning to a partner with expertise in building neurostim technologies. These specialized firms have made the investments in engineering know-how, technology platforms, quality systems, and manufacturing capacity required to build a Class III medical device system like an implantable neurostim device. OEM partners can leverage this strength to supplement and accelerate their development activities.
“We offer one of the broadest portfolios of neurostimulation technologies in the medical device outsource market,” said LaPrade. “This includes full finished device capability for implantable pulse generators (IPGs), implantable leads and external devices (e.g., chargers); critical IPG components, including rechargeable Li-ion and non-rechargeable battery technology, hermetic feedthroughs, formed enclosures, header assemblies, and machined components; and critical lead components, including precious metal electrodes, single wire and multi filar coiled lead wires and molded assemblies.”
Because medical device outsource partners like Integer have engineers with specific knowledge and experience with neurostim products, they can uniquely assist OEMs in refining product concepts and translating requirements into designs and specifications. “During this upfront design phase, our engineers offer a consultative approach and often provide our customers with recommendations for improvements in size, form factor, manufacturability, reliability, performance, and cost,” LaPrade explained.
The faster and more effectively a neurostim device can be built, the quicker patients can utilize this therapy poised to serve as effective treatment for disease states throughout the body.
“We offer comprehensive development services for finished systems, including mechanical, electronics, software, firmware, and process development,” said LaPrade. “We have developed more than 40 IPG systems throughout our 40+ year history. During this time, we have also created a portfolio of platform technology modules that we customize, which significantly reduces the time and risk of development. As a vertically integrated supplier, we can offer custom component (e.g., battery) design and development services.”
The practice has understandably since evolved, and researchers worldwide are investigating altering nerve activity in specific nerves or neurological sites in the body. Applying small, electrical stimuli to specific nerves has been found to have enormous potential in treating various conditions from epilepsy to mental health to heart disease. This method of treating chronic conditions is known as neuromodulation or neurostimulation (neurostim). Eventually, neurostim devices were implanted in the body at specific sites for more direct application of electrical pulses.
“Although originally invasive in nature, neurostimulation devices have evolved from invasive procedures to external electrical stimulators, such as vagus nerve stimulation (VNS) devices to transcutaneous vagus nerve stimulation (TVNS) devices,” said David Novotny, general manager and global head of medical device and diagnostic research for ICON plc, a Dublin, Ireland-based global provider of outsourced development and commercialization services to pharmaceutical, biotechnology, medical device, and government and public health organizations. “Moreover, engineers have focused on reducing battery size in implantable devices, making implantable stimulators a more attractive option for patients concerned with cosmetic appearances.”
The core neurostimulation therapies of spinal cord stimulation, deep brain stimulation, and vagus nerve stimulation have been continuously advancing. For example, spinal cord stimulation was initially centered on hardware innovation and the focus has shifted to waveform innovations, strategies that stimulate the spinal cord by a different mechanism of action like high-frequency or burst stimulation, which consists of closely packed high-frequency impulses, followed by a quiescent period. Other advancements include systems that automatically adjust treatment and shrinking the devices for the patient’s benefit.
“The introduction of more advanced waveforms and stimulation frequencies offers greater programming flexibility to meet patients’ needs,” commented Brian LaPrade, product management director, cardiac rhythm management & neuromodulation at Integer Holdings Corp., a Plano, Texas-based medical device outsource manufacturer. “We also see the development of closed-loop systems that enable devices to automatically adjust based on ‘detected’ responses, miniaturization of devices to improve patient comfort and reduce procedure invasiveness (including use of externally powered ‘injectable’ devices), and a shift to Bluetooth communication between the implanted device and externals.”
The neurostim market has flourished as proof of these devices’ effectiveness has grown. Neurostim’s scientific and technological advancement continues due to the increase in the geriatric population and the rise of the devices’ safety and efficacy. Pamela Kennedy, R.N.—ICON plc’s executive director, business development, medical device and diagnostic research—outlined the myriad external and internal neurostim technologies and how they are impacting various therapeutic areas:
External neurostim devices:
- Transcutaneous electrical nerve stimulation (TENS)—chronic neuropathic pain, fibromyalgia
- Transcutaneous vagus nerve stimulation (TVNS)—anxiety, depression, autism, aging
- Transcranial magnetic stimulation (TMS)—depression, ADHD
- Respiratory electrical stimulation (RES)—Improvement of respiratory function after spinal cord injury
- Spinal cord stimulation (SCS)—pain management, spasticity
- Deep brain stimulation (DBS)—Parkinson’s, depression, epilepsy
- Vagus nerve stimulation (VNS)—epilepsy, depression
- Sacral nerve stimulation (SNS)—urinary incontinence, constipation
- Gastric electrical stimulation (GES)—obesity, gastroparesis
“Neurostimulation technology offers potential to improve upon traditional treatment options in the areas of epilepsy, Parkinson’s, depression, sleep apnea, dystonia (spasticity), pain management, obesity/gastric, migraine, and heart disease,” Kennedy explained. “Also, neurostimulation devices are improving upon traditional treatments for these conditions by offering an affordable and non-invasive treatment option in a financially overburdened healthcare system. Moreover, in the case of Parkinson’s disease, epilepsy, and urinary incontinence conditions, neurostimulation offers hope for patients that previously had little to no treatment options and poor quality of life.”
First used to treat pain in 1967, SCS delivers mild electrical stimulation to the nerves along the spinal column, modifying nerve activity to minimize pain signals to the brain. The therapy began routine use in the 1980s, and the FDA first approved SCS in 1989 to relieve chronic pain from nerve damage in the trunk, arms, or legs. According to the International Neuromodulation Society, SCS therapy accounts for about 70 percent of all neuromodulation treatments, and about 34,000 patients undergo SCS implants each year worldwide.
“While vagus nerve stimulation and deep brain stimulation led the industry in the mid-1990s, spinal cord stimulation continues to hold the largest market share today,” Kennedy noted.
SCS treats pain that is neuropathic in origin—meaning it arises from nerve damage and doesn’t serve a protective purpose. The damage usually occurs as a result of accident, injury, or disease and SCS therapy is most commonly indicated for the treatment of neuropathic back and leg pain. Since the 80s, SCS therapy has advanced in ways that tailor the treatment to a patient’s individual needs. Newer lead designs allow more precise control of the electrical field, and increasingly sophisticated devices offer a myriad of stimulation parameters. Some SCS devices can even achieve pain reduction without evoking perceptible sensations.
Medical device makers featuring a cardiac rhythm management portfolio—Medtronic, Abbott Laboratories, and Boston Scientific, for example—typically also offer SCS and other neurostim devices because both devices’ mechanisms of action similarly involve the delivery of electrical pulses to a specific site via a lead. The latest SCS technologies incorporate peripheral tools to assist in recording/analyzing the treatment and/or target novel neurological areas to enhance therapy. Medtronic claims its Intellis SCS platform with AdaptiveStim is the smallest fully implantable spinal cord neurostimulator and includes a clinician programmer and reporting via tablet to track a patient’s progress. Abbott’s Proclaim DRG targets the dorsal root ganglion (DRG) to stimulate primary sensory neurons, a processing point of sensory information. Boston Scientific claims its Spectra WaveWriter SCS system is the first designed to personalize pain relief through combination therapy and the simplicity of waveform automation.
Rather than blocking signals to the brain, DBS involves implanting electrodes within certain areas of the brain. These electrodes produce electrical impulses that regulate abnormal impulses or affect specific cells and chemicals within the brain. The amount of DBS is controlled by a pacemaker-like device placed under the skin in the upper chest, and a wire traveling under the skin connects it to the electrodes in the brain.
DBS is currently approved by the FDA to treat dystonia (a movement disorder), epilepsy, essential tremor, obsessive-compulsive disorder, and Parkinson’s disease. It is also undergoing evaluation as a potential treatment for a variety of conditions: addiction, chronic pain, cluster headache, dementia, major depressive disorder, Huntington’s disease, multiple sclerosis, stroke recovery, Tourette syndrome, and traumatic brain injury. As the procedure to install a DBS system involves both brain surgery and chest wall surgery, the treatment is reserved for people who aren’t able to control their symptoms with medications.
Modern DBS systems also feature enhancements that improve the clinician and patient interaction with the device. Medtronic’s Activa DBS portfolio contains the first rechargeable device and has 15-year longevity. Abbott’s Infinity DBS system is controlled with an Apple iPad mini mobile digital device so the doctor can adjust the setting for personalized, discreet therapy management. Boston Scientific claims its Vercise DBS system was the first engineered for precise neural targeting to customize therapy for patients with Parkinson’s disease, primary and secondary dystonia, and essential tremor. Its Vercise Gevia DBS system was approved for MRI labeling toward the end of August.
New therapeutic areas have been uncovered as research in neurostim continues. For example, continuous positive airway pressure (CPAP) is the usual treatment option for obstructive sleep apnea (OSA). Many people can’t tolerate the cumbersome nightly therapy. The FDA approved Inspire Upper Airway Stimulation in 2014 to treat OSA in those that can’t tolerate CPAP, and it has found success. Further, VNS, which has historically been used to treat epilepsy, has found success in curbing obesity as well.
“Neurostimulation has been leveraged to treat sleep apnea by stimulating the activity of the hypoglossal nerve, which supports the activity of the tongue,” said Novotny. “In sleep apnea patients, because the tongue relaxes and collapses into the upper airway during sleep, the device stimulates the tongue to help prevent obstructions in the upper airway. Neurostimulation has also been shown to help treat obesity by stimulating the vagus nerve, which serves as the signaling connection between the stomach and the brain.”
Neurostim technology has the potential to be an aid in stemming opioid abuse, as well. The symptoms of opioid withdrawal can be incredibly distressing, come on quickly, and are one of the main reasons patients with physical dependence on the drugs have so much trouble stopping them. Early symptoms usually begin in the first 24 hours depending on the intensity of the patient’s physical dependence on the drug and include anxiety, cramping, muscle aches, restlessness, sweating, tears, and a runny nose. Later, more intense symptoms like diarrhea, abdominal cramping, nausea and vomiting, rapid heartbeat, and hypertension set in after the first day.
Innovative Health Solutions’ (IHS) NSS-2 BRIDGE is a percutaneous nerve field stimulator device system that can be used as an aid to reduce symptoms of opioid withdrawal through application to branches of specific cranial nerves, as well as branches of the occipital nerves identified via transillumination. NSS-2 BRIDGE received FDA clearance in November 2017 and has since been used by doctors and treatment centers at the local and state level to assist thousands of people suffering from opioid withdrawal symptoms.
“The NSS-2 BRIDGE goes behind the patient’s ear and includes micro-needle arrays that percutaneously implant in and around the ear and send gentle electrical impulses to branches of the nerves that lead to central brain regions involved in symptoms of withdrawal, particularly the amygdala and spinal cord,” explained an IHS spokesperson. “Research study results show an 84.6 percent reduction of withdrawal symptoms in as little as 60 minutes.”
The device helps to alleviate the opioid withdrawal symptoms of nausea, diarrhea, vomiting, abdominal pain, anxiety, and restlessness. Relieving these symptoms in as little as 60 minutes can go a long way to aid in a long-term recovery plan from opioid withdrawal.
“Greater early decreases in withdrawal scores have been shown to significantly correlate with long-term treatment success,” the IHS spokesperson went on.
IHS also gained FDA clearance in June for the first treatment indicated to reduce pain in children dealing with functional abdominal pain associated with irritable bowel syndrome (IBS). “There are currently no other approved therapies for IBS in children. Currently approved medications for IBS in adults all work at the level of the gut and are often used off-label in children, despite the risk of serious side effects,” the company spokesperson explained. “Also, the intensity of withdrawal symptoms has been correlated with treatment drop-out. Therefore, treatment with NSS-2 BRIDGE is likely to enhance long-term recovery, regardless of the type of medication-assisted therapy used. It allows patients to overcome a major hurdle and is meant to enhance long-term recovery.”
IB-Stim targets brain regions involved in pain processing, unlike traditional therapies for IBS that target the gut. IB-Stim is also nonsurgical, targeting central brain areas through stimulation of cranial nerves in the ear. Prior to FDA clearance, the device underwent a double-blind, randomized, controlled trial that included 104 patients aged 11-18 with functional abdominal pain disorders, measuring the effects of IB-Stim compared to placebo on several pain aspects including worst pain and the pain-frequency-severity-duration composite score. It also measured patients’ overall symptom improvement based on the Symptom Response Scale. The company’s sub-analysis of IBS subjects showed before IB-Stim treatment, 70 percent of patients failed to improve with four medications trialed. However, patients treated with IB-Stim had an 81 percent improvement in global symptoms with no serious adverse events, and minimal to no side effects.
“IB-Stim treats functional abdominal pain associated with IBS in patients ages 11-18,” the company spokesperson commented. “The IB-Stim is a non-surgical device that is placed behind the patient’s ear during an outpatient visit and works by sending gentle electrical impulses into cranial nerve bundles located in the ear. This stimulation targets brain areas involved in processing pain and aids in the reduction of functional abdominal pain associated with IBS.”
Bioelectronic medicine merges molecular medicine, neuroscience, engineering, and computing to develop devices to diagnose and treat diseases, and represents a potential revolution in disease management. The discipline arose from discoveries of mechanisms for neural control of biological processes that underlie disease, and development of technologies to therapeutically modulate those specific neural circuits using electrons in place of drugs. A bioelectronic medical device uses precise electrical pulses like a neurostim technology, but the pulses are used to activate systemic responses for the treatment of certain chronic diseases.
“Bioelectronic medicine differs from traditional neuromodulation therapies by addressing the underlying pathophysiology to provide a potential cure for the disease versus simply masking the symptoms of disease,” explained Ankit Shah, senior director of commercialization and marketing for SetPoint Medical, a Valencia, Calif.-based bioelectronic medicine company that is dedicated to treating patients with chronic autoimmune diseases.
SetPoint Medical’s unique approach to bioelectronic medicine aims to return the body to its natural immunological “set point” by using vagus nerve stimulation to restore immunological homeostasis. It does so through targeted electrical pulses to the vagus nerve to trigger the inflammatory reflex, which regulates and restores balance to the immune system. The vagus nerve also innervates the spleen, which is also responsible for responding to infection and inflammation. Activating the inflammatory reflex alters the phenotype of inflammatory cells in the spleen, reducing the amount of circulating cytokines and dampening systemic inflammation.
Current vagus nerve stimulating devices require two incisions to implant a large pulse generator in the chest with a wired lead tunneled to the neck. SetPoint’s device, however, is a miniaturized, rechargeable system with an integrated electrode and no lead, so it can be implanted with a single incision, minimally invasive procedure on the nerve. This reduction in the implant’s footprint substantially benefits patients, delivering a proprietary pulse pattern for autoimmune disease treatment.
“We have conducted multiple clinical studies to better understand the safety and effectiveness of bioelectronic medicine as a potential alternative to treatment of chronic, inflammation-mediated autoimmune diseases,” said Shah. “We are initially focused on the development of our device for the treatment of rheumatoid arthritis (RA) as a lead indication. Our device also has promising data for Crohn’s disease as well as multiple sclerosis (MS) in various stages of development.”
RA is a challenging condition to treat because according to SetPoint, 30-40 percent of RA patients don’t adequately respond to or are intolerant of biologic therapies—those therapies already have potentially serious side effects, and patient compliance is a serious issue. These biologic therapies also financially burden the healthcare system because some of the highest revenue grossing drugs are indicated for patients with RA and other autoimmune diseases.
“There is a major unmet need for novel treatment options for autoimmune diseases that are more efficacious, less immunosuppressive, and more cost-effective than currently approved therapies,” noted Shah.
The company recently posted positive results from its U.S. pilot IDE study evaluating its bioelectronic medicine device for RA treatment. SetPoint Medical’s bioelectronic medicine was found to be well-tolerated with no device-related adverse events through 12 weeks. Five of the 10 active stimulation patients who had failed multiple biologic and targeted synthetic therapies had clinically meaningful improvement in RA signs and symptoms as measured by Disease Activity Score (DAS28-CRP) and Clinical Disease Activity Index (DCAI), two validated disease measurement instruments. Two of those patients even achieved DAS28-CRP remission.
“Unlike traditional biologic therapies which aim to eliminate the bioavailability of a single pro-inflammatory cytokine, SetPoint’s bioelectronic medicine approach has been shown to suppress a range of inflammatory cytokines by 30-70 percent without eliminating the bioavailability of any single cytokine,” said Shah. “In doing so, inflammation can be reduced without causing significant immunosuppression, which is a major risk factor for serious side effects like systemic infections associated with almost all currently available therapies for RA.”
In addition to clinical studies in RA and Crohn’s disease, SetPoint is currently studying the potential of its bioelectronic medicine for MS. Preclinical research is ongoing, and according to the company, results show promising early signals of efficacy in animal MS models.
Neurostim OEMs, like any other medical device maker, aim to bring their innovative therapies to market as fast and as cost-effectively as possible. When this challenge involves more capacity and cost than the device maker has, they might consider turning to a partner with expertise in building neurostim technologies. These specialized firms have made the investments in engineering know-how, technology platforms, quality systems, and manufacturing capacity required to build a Class III medical device system like an implantable neurostim device. OEM partners can leverage this strength to supplement and accelerate their development activities.
“We offer one of the broadest portfolios of neurostimulation technologies in the medical device outsource market,” said LaPrade. “This includes full finished device capability for implantable pulse generators (IPGs), implantable leads and external devices (e.g., chargers); critical IPG components, including rechargeable Li-ion and non-rechargeable battery technology, hermetic feedthroughs, formed enclosures, header assemblies, and machined components; and critical lead components, including precious metal electrodes, single wire and multi filar coiled lead wires and molded assemblies.”
Because medical device outsource partners like Integer have engineers with specific knowledge and experience with neurostim products, they can uniquely assist OEMs in refining product concepts and translating requirements into designs and specifications. “During this upfront design phase, our engineers offer a consultative approach and often provide our customers with recommendations for improvements in size, form factor, manufacturability, reliability, performance, and cost,” LaPrade explained.
The faster and more effectively a neurostim device can be built, the quicker patients can utilize this therapy poised to serve as effective treatment for disease states throughout the body.
“We offer comprehensive development services for finished systems, including mechanical, electronics, software, firmware, and process development,” said LaPrade. “We have developed more than 40 IPG systems throughout our 40+ year history. During this time, we have also created a portfolio of platform technology modules that we customize, which significantly reduces the time and risk of development. As a vertically integrated supplier, we can offer custom component (e.g., battery) design and development services.”