Maria Shepherd, President and Founder, Medi-Vantage07.30.19
With commanding new players like Apple and Google entering the healthcare space, the continued surge of mergers and acquisitions, vertical integrations, and no clear U.S. healthcare policy and reform direction for our $3.6 trillion annual healthcare spend,1 the healthcare ecosystem remains in a phase of dramatic disruption.
What Is Keeping Hospital CEOs Awake at Night?
A study by the American College of Healthcare Executives (ACHE)2 involving community hospital CEOs (n=355) shows several common themes hospital CEOs grapple with today. Several of them, including the top issues outlined in Table 1, offer opportunities where medtech can help.
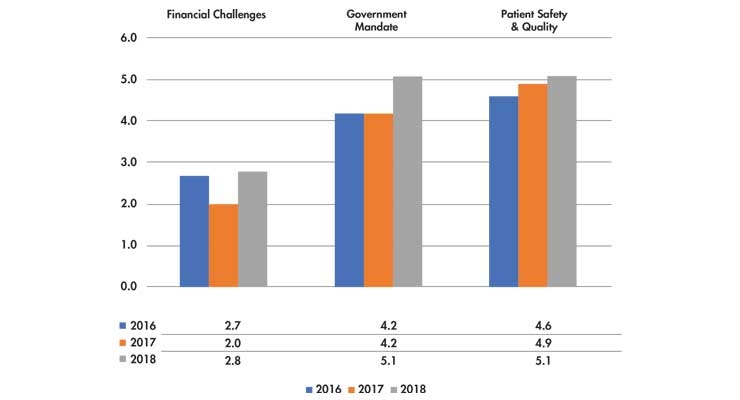
Table 1: Rank of CEOs’ top concerns over issues affecting their hospitals (on a scale of 1-10, with 1 representing the highest level of concern)2
The ACHE survey indicates hospital leadership is working to address the challenges of managing limited reimbursements against the rising costs of bringing talented staff (and retaining them) to provide the best possible care for their patients. Also indicated as concerning issues in the study were Personnel Shortages (#4), Behavioral Health/Addiction (#5), Patient Satisfaction (#6), Access to Care (#7), Physician-Hospital Relations (#8), Technology (#9), Population Health Management (#10) and Reorganization (#11).
Medtech to the Rescue
There are many drivers in the healthcare system that apply cost pressure to hospital margins. Seventy percent of hospital CEOs are concerned that CMS regulations are a moving target.2 In addition to the obvious root causes, an AHA study3 predicts U.S. healthcare systems will lose $218 billion more in federal reimbursement payments by 2028, and private payers plan to follow the trend.
Medical devices that aid in reducing hospital readmission rates help hospitals retain CMS reimbursement and address a major financial challenge for hospitals. Penalties for readmissions are harsh, especially with patients admitted for heart failure, heart attack, or pneumonia. The issue can drop CMS reimbursement by as much as 3 percent if readmission rates are too high. Private payers are also implementing penalty and credit models similar to those of CMS, and hospitals are motivated to control readmission rates so they may achieve a superior negotiating position with payers to improve their rates or to become a Center of Excellence. Beyond concerns over reimbursement penalties, readmissions also drive up hospital costs, impacting overall profitability.
One medical device company helping hospitals to reduce readmission rates is Avanos.4 Rather than use opioids in the OR, Avanos recommends helping patients manage pain in surgery using continuous peripheral nerve blocks. Once the surgery is over, the patient’s pain is managed through a postoperative pain pump, such as the ON-Q Pain Relief System. This combination of therapies offers patients pain relief without the side effects of traditional methods for surgical pain management, such as nausea, vomiting, grogginess, and the long-term potential for addiction.
Managing Value-Based Care
Since 2011, the number of accountable care organizations (ACOs) has grown5 (Table 2), but most healthcare organizations still rely on fee-for-service as their greatest source of income.
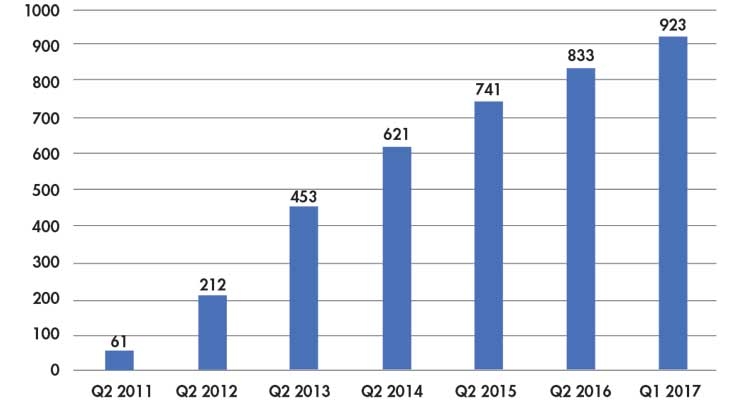
Table 2: Growth in number of ACOs5
One product that will help ACO and fee-for-service hospitals maintain or reduce costs is the Medtronic Tyrx Antibacterial Envelope.6 Infected heart devices must be replaced, and this adds greater than $1 billion in annual expense to the U.S. healthcare system. Medtronic has inked agreements with more than 140 U.S. hospitals in an effort to reduce infection rates in pacemakers and ICDs using the Tyrx dissolvable surgical envelopes.
Medtronic is so confident in the Tyrx product’s effectiveness in reducing infection rates that the organization is signing risk-sharing contracts with healthcare providers. These agreements state the firm will pay a rebate toward the estimated $50,000 price tag to remove an infected Medtronic heart device and cost of implanting a new device if the Tyrx product is used in the original surgery and results in an infection.6 Cardiac implantable electronic device infections generally occur in two categories: pocket infection and systemic infection (Table 3). While the true incidence of infection is hard to capture in the clinical literature, it is generally estimated to be between 2 and 4 percent.8
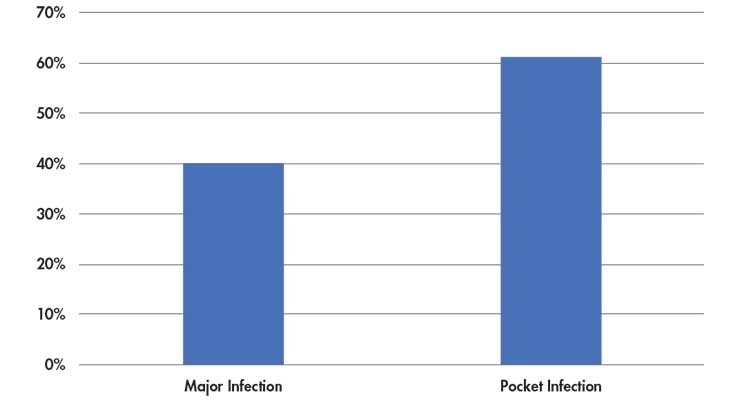
Table 3: Percentage reduction of infection from the use of Medtronic’s Tyrx envelope in cardiac implant surgery7
The Medi-Vantage Perspective
The Tyrx device was launched in Europe in September 2014 and is now available for use with implantable neurostimulators. What tools are in your company’s bag that can be engineered to help address what’s keeping hospital CEOs up at night?
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.
What Is Keeping Hospital CEOs Awake at Night?
A study by the American College of Healthcare Executives (ACHE)2 involving community hospital CEOs (n=355) shows several common themes hospital CEOs grapple with today. Several of them, including the top issues outlined in Table 1, offer opportunities where medtech can help.

Table 1: Rank of CEOs’ top concerns over issues affecting their hospitals (on a scale of 1-10, with 1 representing the highest level of concern)2
The ACHE survey indicates hospital leadership is working to address the challenges of managing limited reimbursements against the rising costs of bringing talented staff (and retaining them) to provide the best possible care for their patients. Also indicated as concerning issues in the study were Personnel Shortages (#4), Behavioral Health/Addiction (#5), Patient Satisfaction (#6), Access to Care (#7), Physician-Hospital Relations (#8), Technology (#9), Population Health Management (#10) and Reorganization (#11).
Medtech to the Rescue
There are many drivers in the healthcare system that apply cost pressure to hospital margins. Seventy percent of hospital CEOs are concerned that CMS regulations are a moving target.2 In addition to the obvious root causes, an AHA study3 predicts U.S. healthcare systems will lose $218 billion more in federal reimbursement payments by 2028, and private payers plan to follow the trend.
Medical devices that aid in reducing hospital readmission rates help hospitals retain CMS reimbursement and address a major financial challenge for hospitals. Penalties for readmissions are harsh, especially with patients admitted for heart failure, heart attack, or pneumonia. The issue can drop CMS reimbursement by as much as 3 percent if readmission rates are too high. Private payers are also implementing penalty and credit models similar to those of CMS, and hospitals are motivated to control readmission rates so they may achieve a superior negotiating position with payers to improve their rates or to become a Center of Excellence. Beyond concerns over reimbursement penalties, readmissions also drive up hospital costs, impacting overall profitability.
One medical device company helping hospitals to reduce readmission rates is Avanos.4 Rather than use opioids in the OR, Avanos recommends helping patients manage pain in surgery using continuous peripheral nerve blocks. Once the surgery is over, the patient’s pain is managed through a postoperative pain pump, such as the ON-Q Pain Relief System. This combination of therapies offers patients pain relief without the side effects of traditional methods for surgical pain management, such as nausea, vomiting, grogginess, and the long-term potential for addiction.
Managing Value-Based Care
Since 2011, the number of accountable care organizations (ACOs) has grown5 (Table 2), but most healthcare organizations still rely on fee-for-service as their greatest source of income.

Table 2: Growth in number of ACOs5
One product that will help ACO and fee-for-service hospitals maintain or reduce costs is the Medtronic Tyrx Antibacterial Envelope.6 Infected heart devices must be replaced, and this adds greater than $1 billion in annual expense to the U.S. healthcare system. Medtronic has inked agreements with more than 140 U.S. hospitals in an effort to reduce infection rates in pacemakers and ICDs using the Tyrx dissolvable surgical envelopes.
Medtronic is so confident in the Tyrx product’s effectiveness in reducing infection rates that the organization is signing risk-sharing contracts with healthcare providers. These agreements state the firm will pay a rebate toward the estimated $50,000 price tag to remove an infected Medtronic heart device and cost of implanting a new device if the Tyrx product is used in the original surgery and results in an infection.6 Cardiac implantable electronic device infections generally occur in two categories: pocket infection and systemic infection (Table 3). While the true incidence of infection is hard to capture in the clinical literature, it is generally estimated to be between 2 and 4 percent.8

Table 3: Percentage reduction of infection from the use of Medtronic’s Tyrx envelope in cardiac implant surgery7
The Medi-Vantage Perspective
The Tyrx device was launched in Europe in September 2014 and is now available for use with implantable neurostimulators. What tools are in your company’s bag that can be engineered to help address what’s keeping hospital CEOs up at night?
References
- http://bit.ly/mpo190701 [PDF]
- http://bit.ly/mpo190702
- http://bit.ly/mpo190703 [PDF]
- http://bit.ly/mpo190704 [PDF]
- http://bit.ly/mpo190705
- http://bit.ly/mpo190706
- http://bit.ly/mpo190707
- http://bit.ly/mpo190708
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.