Mihir Torsekar, Analyst, U.S. International Trade Commission07.30.19
The United States is widely considered to have developed the world’s leading medtech industry, consistently ranking among the highest in R&D spending, registration of international patents, and sales. Much of this success owes to the strategic investments U.S. medtech multinationals have made in key international markets, which have contributed to the development of “clusters,” a term that describes the close collaboration that occurs between companies, suppliers, similar industries, research, and institutions working in close proximity to develop innovative products.1
For the most part, U.S. companies began investing in these clusters during the 1990s in response to low labor costs associated with these destinations, their duty-free trading relationship, and various other incentives. As such, these clusters have enabled U.S. medtech investors to maintain their internationally competitive position by lowering overall production costs and affording access to burgeoning or established markets. This article presents three key takeaways from four such clusters: China, Costa Rica, Ireland, and Mexico.
Each Cluster Is Export-Oriented
Despite representing underserved medtech markets—all four clusters rank relatively low in terms of per capita spending on medical devices—most medtech clusters are export-oriented, serving larger, developed markets. The United States is the largest single-country recipient of medical goods from each of these clusters. For example, more than 90 percent of Mexico’s medtech production has been exported, with the United States receiving nearly all (94 percent or $9.5 billion in 2018) of these exports. Likewise, the United States receives roughly 60 percent of Costa Rica’s medtech exports.
With the hopes of accessing the EU market, U.S. medtech investments in Ireland over the past decade have outpaced that of any other cluster discussed in this article—roughly two-thirds of Ireland’s medtech production has been exported to the trading bloc in recent years.
China’s Domestic Market—A Principal Driver of U.S. Investment
In contrast to the medtech clusters in Costa Rica, Ireland, and Mexico, where investments by multinationals are principally intended to serve the U.S. or EU market, U.S. presence in China is also heavily directed toward supplying the local market. In particular, with slow projected medical device market growth in developed markets (U.S., EU, and Japan), China has emerged as one of the most promising options for medtech firms. Although the United States remains heavily reliant on medtech imports from China, the country is also the third-largest export market for the United States.
China’s sizeable market remains undersupplied and the growing incidence of afflictions ranging from non-communicable diseases (cardiovascular disease, lung cancer, and diabetes) to musculoskeletal degradations among the country’s aging population is presenting substantial opportunities for U.S. medtech firms. At the same time, China has increased its domestic healthcare spending and has made progress in reforming its regulatory structure in accordance with international best practices.
Incentives Matter
In addition to capitalizing on low-labor costs commonly found in medtech clusters, U.S. medtech investments have largely corresponded to substantial incentives. For example, the vast majority of all U.S. investments made in the clusters discussed in this article were in cities where free-trade zones (FTZs) are located. FTZs encourage foreign investment by extending duty-free treatment on imports and exports to foreign manufacturers, granting exemptions on excise taxes, and providing income tax benefits.
In the case of Mexico, U.S. foreign direct investment (FDI) into the medtech sector has been abetted by the existence of FTZs (referred to as “maquiladoras”), as well as the passage of the North American Free Trade Agreement (NAFTA). In the 25 years since NAFTA’s creation, Mexico has emerged as perhaps the most important medtech cluster for the United States, serving as its leading supplier over the past decade.
At the same time, Mexico has implemented policy measures to attract FDI—i.e., the 2005 Law of Competitiveness and Economic Development of Baja California. Further, Mexico has unveiled measures to ease the cost of doing business and established technical schools to train the local workforce to fulfill the industry’s requirements—all of which has further encouraged U.S. investments into the medtech sector.
Costa Rica’s emergence as a vital supplier for U.S. medtech manufacturing has principally been driven by a low-cost labor force, but also from FTZs where generous income tax exemptions are granted; an estimated 90 percent of foreign firms operate in these areas.
With regard to Ireland, the country’s proximity to and duty-free treatment with the EU market have translated into substantial investments by U.S. medtech firms. In addition, the presence of notified bodies within Ireland has facilitated regulatory compliance with EU standards. At the same time, local investment promotion agencies, coupled with government loan guarantees to foreign firms, have further facilitated investment.
Foreign investment in China’s medtech sector has generally been encouraged through wholly-owned subsidiaries operating within the country or by establishing joint ventures with local Chinese firms. Notably, the investment landscape has been challenging to navigate, but the presence of FTZs has encouraged U.S. investment.
Clusters Help Connect Different Elements of the Value Chain
Value chains refer to the iterative steps taken to transform a product from conception to consumption, and they are broadly divided into low and high value-added activities. Generally speaking, manufacturing and assembly contribute the lowest value to the overall production process, while services such as R&D and distribution are the highest (Figure 1).

Figure 1: Example of a value chain.
By establishing international medtech clusters, U.S. firms have been able to specialize in high value-added activities such as R&D and marketing, while allowing other countries to manufacture components and perform low-cost assembly. As such, the majority of U.S. investments into these four clusters over the past decade have prioritized manufacturing.
Ireland and China are Climbing the Value Chain
Although investments in Ireland and China initially were led by low-end manufacturing, over the past decade or so, U.S. medtech investments in these countries have been increasingly directed toward higher value-added activities such as R&D, design, product development, and testing. This suggests both Ireland and China have been ascending along the global value chain. Ireland, for example, led all the other clusters in receiving roughly $682 million worth of such investments during 2009–2019, as U.S. firms increasingly relocated R&D operations closer to their Irish manufacturing operations beginning in the late 2000s. A similar pattern has emerged in China, which received an estimated $539 million during the past decade from U.S. medtech firms (Figure 2).
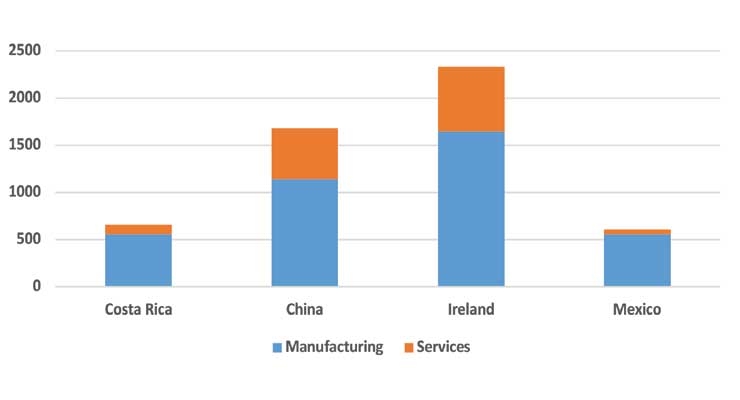
Figure 2: U.S. medtech FDI to four notable clusters during 2009-2018 (millions $). Source: FDI Intelligence.
In addition to the expansion of investments directed to higher value-added activities, both China and Ireland have developed homegrown solutions to medical device manufacturing, with local firms producing a growing share of these goods. For example, Irish-owned firms produce the vast majority of components used in the production of medical devices. Similarly, China’s domestic medtech companies are quickly building domestic market share. Mindray and Wego—two of China’s leading domestic medtech companies (by sales)—achieved a compound annual growth rate of 16 percent and 19 percent respectively from 2010–2015, according to a recent Boston Consulting Group report.
At the same time, collaborations between both of those local firms and U.S. multinationals have increased the value of medtech exports. For both China and Ireland, the leading category of medtech exports are therapeutic devices, which are medium to high-tech goods.
Costa Rica and Mexico Largely Remain Low-Value Added Players
In contrast to China and Ireland, Costa Rica and Mexico have remained engaged in the low-value added elements of the value chain; production within these two clusters largely consists of components and other manufacturing of low value. For example, the majority of medical devices produced within these clusters are disposables and surgical instruments clusters and manufacturing is almost entirely conducted by U.S. affiliates, as opposed to indigenous firms; roughly 90 percent of U.S. medtech imports from Costa Rica are from their local affiliates, according to the U.S. Census Bureau. During 2009–2018, U.S. multinationals have invested roughly $656 million into the country, with nearly 85 percent of this being directed towards manufacturing. v
Reference
Mihir Torsekar covers trade and competitiveness issues affecting the U.S. and global medical device industry as part of his duties at the United States International Trade Commission.
For the most part, U.S. companies began investing in these clusters during the 1990s in response to low labor costs associated with these destinations, their duty-free trading relationship, and various other incentives. As such, these clusters have enabled U.S. medtech investors to maintain their internationally competitive position by lowering overall production costs and affording access to burgeoning or established markets. This article presents three key takeaways from four such clusters: China, Costa Rica, Ireland, and Mexico.
Each Cluster Is Export-Oriented
Despite representing underserved medtech markets—all four clusters rank relatively low in terms of per capita spending on medical devices—most medtech clusters are export-oriented, serving larger, developed markets. The United States is the largest single-country recipient of medical goods from each of these clusters. For example, more than 90 percent of Mexico’s medtech production has been exported, with the United States receiving nearly all (94 percent or $9.5 billion in 2018) of these exports. Likewise, the United States receives roughly 60 percent of Costa Rica’s medtech exports.
With the hopes of accessing the EU market, U.S. medtech investments in Ireland over the past decade have outpaced that of any other cluster discussed in this article—roughly two-thirds of Ireland’s medtech production has been exported to the trading bloc in recent years.
China’s Domestic Market—A Principal Driver of U.S. Investment
In contrast to the medtech clusters in Costa Rica, Ireland, and Mexico, where investments by multinationals are principally intended to serve the U.S. or EU market, U.S. presence in China is also heavily directed toward supplying the local market. In particular, with slow projected medical device market growth in developed markets (U.S., EU, and Japan), China has emerged as one of the most promising options for medtech firms. Although the United States remains heavily reliant on medtech imports from China, the country is also the third-largest export market for the United States.
China’s sizeable market remains undersupplied and the growing incidence of afflictions ranging from non-communicable diseases (cardiovascular disease, lung cancer, and diabetes) to musculoskeletal degradations among the country’s aging population is presenting substantial opportunities for U.S. medtech firms. At the same time, China has increased its domestic healthcare spending and has made progress in reforming its regulatory structure in accordance with international best practices.
Incentives Matter
In addition to capitalizing on low-labor costs commonly found in medtech clusters, U.S. medtech investments have largely corresponded to substantial incentives. For example, the vast majority of all U.S. investments made in the clusters discussed in this article were in cities where free-trade zones (FTZs) are located. FTZs encourage foreign investment by extending duty-free treatment on imports and exports to foreign manufacturers, granting exemptions on excise taxes, and providing income tax benefits.
In the case of Mexico, U.S. foreign direct investment (FDI) into the medtech sector has been abetted by the existence of FTZs (referred to as “maquiladoras”), as well as the passage of the North American Free Trade Agreement (NAFTA). In the 25 years since NAFTA’s creation, Mexico has emerged as perhaps the most important medtech cluster for the United States, serving as its leading supplier over the past decade.
At the same time, Mexico has implemented policy measures to attract FDI—i.e., the 2005 Law of Competitiveness and Economic Development of Baja California. Further, Mexico has unveiled measures to ease the cost of doing business and established technical schools to train the local workforce to fulfill the industry’s requirements—all of which has further encouraged U.S. investments into the medtech sector.
Costa Rica’s emergence as a vital supplier for U.S. medtech manufacturing has principally been driven by a low-cost labor force, but also from FTZs where generous income tax exemptions are granted; an estimated 90 percent of foreign firms operate in these areas.
With regard to Ireland, the country’s proximity to and duty-free treatment with the EU market have translated into substantial investments by U.S. medtech firms. In addition, the presence of notified bodies within Ireland has facilitated regulatory compliance with EU standards. At the same time, local investment promotion agencies, coupled with government loan guarantees to foreign firms, have further facilitated investment.
Foreign investment in China’s medtech sector has generally been encouraged through wholly-owned subsidiaries operating within the country or by establishing joint ventures with local Chinese firms. Notably, the investment landscape has been challenging to navigate, but the presence of FTZs has encouraged U.S. investment.
Clusters Help Connect Different Elements of the Value Chain
Value chains refer to the iterative steps taken to transform a product from conception to consumption, and they are broadly divided into low and high value-added activities. Generally speaking, manufacturing and assembly contribute the lowest value to the overall production process, while services such as R&D and distribution are the highest (Figure 1).

Figure 1: Example of a value chain.
By establishing international medtech clusters, U.S. firms have been able to specialize in high value-added activities such as R&D and marketing, while allowing other countries to manufacture components and perform low-cost assembly. As such, the majority of U.S. investments into these four clusters over the past decade have prioritized manufacturing.
Ireland and China are Climbing the Value Chain
Although investments in Ireland and China initially were led by low-end manufacturing, over the past decade or so, U.S. medtech investments in these countries have been increasingly directed toward higher value-added activities such as R&D, design, product development, and testing. This suggests both Ireland and China have been ascending along the global value chain. Ireland, for example, led all the other clusters in receiving roughly $682 million worth of such investments during 2009–2019, as U.S. firms increasingly relocated R&D operations closer to their Irish manufacturing operations beginning in the late 2000s. A similar pattern has emerged in China, which received an estimated $539 million during the past decade from U.S. medtech firms (Figure 2).

Figure 2: U.S. medtech FDI to four notable clusters during 2009-2018 (millions $). Source: FDI Intelligence.
In addition to the expansion of investments directed to higher value-added activities, both China and Ireland have developed homegrown solutions to medical device manufacturing, with local firms producing a growing share of these goods. For example, Irish-owned firms produce the vast majority of components used in the production of medical devices. Similarly, China’s domestic medtech companies are quickly building domestic market share. Mindray and Wego—two of China’s leading domestic medtech companies (by sales)—achieved a compound annual growth rate of 16 percent and 19 percent respectively from 2010–2015, according to a recent Boston Consulting Group report.
At the same time, collaborations between both of those local firms and U.S. multinationals have increased the value of medtech exports. For both China and Ireland, the leading category of medtech exports are therapeutic devices, which are medium to high-tech goods.
Costa Rica and Mexico Largely Remain Low-Value Added Players
In contrast to China and Ireland, Costa Rica and Mexico have remained engaged in the low-value added elements of the value chain; production within these two clusters largely consists of components and other manufacturing of low value. For example, the majority of medical devices produced within these clusters are disposables and surgical instruments clusters and manufacturing is almost entirely conducted by U.S. affiliates, as opposed to indigenous firms; roughly 90 percent of U.S. medtech imports from Costa Rica are from their local affiliates, according to the U.S. Census Bureau. During 2009–2018, U.S. multinationals have invested roughly $656 million into the country, with nearly 85 percent of this being directed towards manufacturing. v
Reference
- David, Semanik, and Torsekar. “Framework for Analyzing the Competitiveness of Advanced Technology Manufacturing Firms,” September 2018.
Mihir Torsekar covers trade and competitiveness issues affecting the U.S. and global medical device industry as part of his duties at the United States International Trade Commission.