Melonie Warfel, Vice President, Medical Device & Diagnostics, Veeva Systems01.30.19
The new Medical Device Regulation (MDR) is changing the rules for medical device manufacturers and distributors selling and marketing in Europe. The MDR replaces the current Medical Devices Directive (MDD) and goes into effect in less than 18 months. The MDR is one of the most significant regulatory changes for medical device manufacturers in decades (Figure 1). Yet, a recent survey revealed the new directive is not well understood. Less than one-third of organizations have a strong comprehension of the MDR and 41 percent have yet to understand the long-term maintenance needed to comply.1
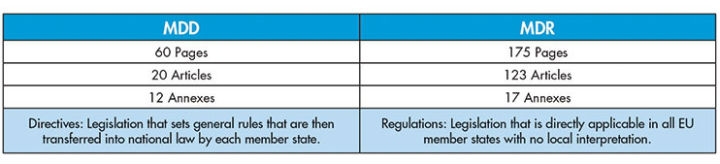
Figure 1: A side-by-side comparison of MDD and the new MDR
Companies face a number of challenges with MDR compliance that were not a factor with MDD. Nearly 60 percent of medical device manufacturers say clinical data management rules are the most challenging component of the new regulation. Additionally, an equal percentage of companies have not yet implemented a strategy to address these MDR clinical requirements.2 It’s now urgent to take appropriate measures to guarantee business continuity and minimize risk. New technology will play a crucial role in helping manufacturers prepare for the MDR and maintain ongoing compliance.
Since the MDR will require manufacturers and distributors to demonstrate clinical evidence regarding the safety of every Class III device in their portfolio, they will need to maintain systems and documentation in permanent compliance throughout the device lifecycle. Close alignment between various fields will be critical, including risk management, clinical evaluation, post-market surveillance, and clinical studies.
It’s no secret that gaining a CE mark—required for any device marketed in any EU member state—will become more difficult under the MDR. For instance, in the past, non-implantable medical devices considered low risk could be “self-marked,” meaning the manufacturer itself simply certifies compliance and applies a CE mark. This will no longer be the case with the MDR.
In order to comply with the new MDR, companies will be required to have greater documentation—most of which will need to be generated from scratch and be easily accessible to health authorities. Significantly, this requires companies to conduct new clinical studies, impacting costs, processes, outsourcing strategy, and even organizational design. The EU health authority did not require new trials for every new product under the MDD—only to provide clinical evidence that the device uses similar materials to those already on the market or delivers the same level of effectiveness as a competitor’s product. With the MDR’s focus on real-world clinical evidence, this approach is no longer compliant.
“New medical device regulations will require a significant change in operating processes for global medical device companies or anyone that sells into Europe,” said Andrew Tummon, director of global clinical affairs at Integra LifeSciences Corporation, a global provider of medical technology, including orthopedic products, surgical instruments, and neurosurgical devices, implants, instruments, and systems.
The May 2020 deadline of the MDR means the clock is ticking for device manufacturers to implement the necessary processes and new technologies for compliance. Following are four steps to help ensure compliance.
1. Conduct a Full Portfolio Review
Companies need to assess their entire portfolio to ensure every device has the necessary clinical evidence available, regardless of when it was introduced or whether it required evidence at the time. Supporting information—such as post-market surveillance plans and reports, post-market clinical follow-up reports, periodic safety update reports, and summaries of safety and clinical performance—must all be in place.
After a full portfolio review, manufacturers and suppliers will be able to identify gaps in the evidence required for MDR compliance. For devices without the supporting clinical evidence, companies may need to conduct a cost-benefit analysis to answer this key question: Is the profit generated by the device enough to justify the expense of a new clinical study?
2. Assess the MDR Gaps
 Manufacturers are responsible for ensuring their products meet the new regulations. The next step is to assess the impact of the MDR on the business, focusing on the discrepancies between the current state and the new criteria. For example, the MDR is much more prescriptive regarding technical documentation and how it must be presented, organized, and made searchable (See Gap Assessment Checklist).
Manufacturers are responsible for ensuring their products meet the new regulations. The next step is to assess the impact of the MDR on the business, focusing on the discrepancies between the current state and the new criteria. For example, the MDR is much more prescriptive regarding technical documentation and how it must be presented, organized, and made searchable (See Gap Assessment Checklist).
3. Evaluate Ability to Capture Clinical Evidence
Gathering clinical evidence under the MDR will have a major impact on how device products are designed, tested, and marketed. Most significantly, it will require companies to conduct more clinical trials. This will require a large investment in resources to capture and store more data completely and transparently. Many medical device manufacturers still use legacy systems that limit their ability to keep up with the size, quantity, and complexity of today’s clinical trials, and this challenge is multiplied with the requirements of the MDR.
4. Implementation and Ongoing Compliance
Medical device manufacturers will have to consider how to allot time and resources to support MDR. Securing executive management support is essential to the success of the program. New educational initiatives can help management comprehend the MDR’s magnitude of change and impact on the business. Beyond the initial implementation phase, manufacturers will need to establish ongoing compliance activities to support the more stringent requirements around clinical evaluation, quality management, and post-market surveillance data management.
A Catalyst for Change
Even as device manufacturers bring remarkable innovations to market, they have largely relied on a combination of manual, paper-based processes and on-premise legacy systems with email for information sharing. These existing systems and processes will struggle in the post-MDR world. Consequently, the MDR may be the catalyst that prompts forward-thinking companies to improve their systems and adopt modern technology.
In many ways, the device landscape mirrors the experience of pharmaceutical companies five to 10 years ago. At that time, changing market conditions led to dramatic transformation across the industry. “Just as pharma realized when they updated enterprise technology, the device industry can benefit from new cloud platforms that allow companies to manage more with less,” added Tummon.
The cloud enables more transparent document and data management across all functions. Silos between departments crumble, increasing visibility into workflows and document lifecycles. In addition, real-time communication and collaboration with internal and external stakeholders, including regulatory authorities, improves dramatically.
Modern systems make regulatory compliance easier to achieve and maintain. Tasks are automatically documented throughout the entire product lifecycle, establishing an audit trail from the moment a record enters a system until archival. With built-in metrics, companies can generate reports for both internal and external use and capture valuable insights on performance.
“Advanced technology becomes critical as we are forced to conduct more clinical studies,” Tummon added. “It’s not sustainable to hire more and more people to manage increased numbers of studies. At Integra, we want to completely eliminate paper and paper-based processes, as well as automate and generate reports at the press of a button. With streamlined processes and capabilities to complete tasks in real time, from anywhere, we can optimize staff time and improve execution.”
The MDR promises to disrupt existing processes for device manufacturers and distributors globally. It will add a new layer of compliance requirements and force more clinical testing of existing and new products. Achieving compliance requires proper preparation, but it will still be difficult—even more so with outdated technology. Adopting new solutions that are flexible, transparent, and scalable will not only help ensure MDR compliance, but also improve overall efficiency and innovation that is crucial to success in any regulatory environment.
References
1 Emergo Outlook Survey 2018 (February 2018).
2 Emergo Blog (October 10, 2018) “Medical Device Companies Underprepared for MDR,” by Stewart Eisenhart.
Melonie Warfel has more than 25 years of technology experience, with deep expertise across multiple verticals—including life sciences, healthcare, manufacturing, and government. At Veeva Systems, Warfel is responsible for global business development and product strategy for the medical devices market, including development and expansion, solution identification, and go-to-market activities. Prior to joining Veeva, Warfel launched and grew Pegasystems’ Life Sciences Business Unit, leveraging the Pega Platform to identify and develop solutions across R&D and commercial. She joined Pegasystems following 19 years with Adobe Systems, where she drove the development of the company’s vertical business strategy. Warfel can be reached at melonie.warfel@veeva.com.

Figure 1: A side-by-side comparison of MDD and the new MDR
Companies face a number of challenges with MDR compliance that were not a factor with MDD. Nearly 60 percent of medical device manufacturers say clinical data management rules are the most challenging component of the new regulation. Additionally, an equal percentage of companies have not yet implemented a strategy to address these MDR clinical requirements.2 It’s now urgent to take appropriate measures to guarantee business continuity and minimize risk. New technology will play a crucial role in helping manufacturers prepare for the MDR and maintain ongoing compliance.
Since the MDR will require manufacturers and distributors to demonstrate clinical evidence regarding the safety of every Class III device in their portfolio, they will need to maintain systems and documentation in permanent compliance throughout the device lifecycle. Close alignment between various fields will be critical, including risk management, clinical evaluation, post-market surveillance, and clinical studies.
It’s no secret that gaining a CE mark—required for any device marketed in any EU member state—will become more difficult under the MDR. For instance, in the past, non-implantable medical devices considered low risk could be “self-marked,” meaning the manufacturer itself simply certifies compliance and applies a CE mark. This will no longer be the case with the MDR.
In order to comply with the new MDR, companies will be required to have greater documentation—most of which will need to be generated from scratch and be easily accessible to health authorities. Significantly, this requires companies to conduct new clinical studies, impacting costs, processes, outsourcing strategy, and even organizational design. The EU health authority did not require new trials for every new product under the MDD—only to provide clinical evidence that the device uses similar materials to those already on the market or delivers the same level of effectiveness as a competitor’s product. With the MDR’s focus on real-world clinical evidence, this approach is no longer compliant.
“New medical device regulations will require a significant change in operating processes for global medical device companies or anyone that sells into Europe,” said Andrew Tummon, director of global clinical affairs at Integra LifeSciences Corporation, a global provider of medical technology, including orthopedic products, surgical instruments, and neurosurgical devices, implants, instruments, and systems.
The May 2020 deadline of the MDR means the clock is ticking for device manufacturers to implement the necessary processes and new technologies for compliance. Following are four steps to help ensure compliance.
1. Conduct a Full Portfolio Review
Companies need to assess their entire portfolio to ensure every device has the necessary clinical evidence available, regardless of when it was introduced or whether it required evidence at the time. Supporting information—such as post-market surveillance plans and reports, post-market clinical follow-up reports, periodic safety update reports, and summaries of safety and clinical performance—must all be in place.
After a full portfolio review, manufacturers and suppliers will be able to identify gaps in the evidence required for MDR compliance. For devices without the supporting clinical evidence, companies may need to conduct a cost-benefit analysis to answer this key question: Is the profit generated by the device enough to justify the expense of a new clinical study?
2. Assess the MDR Gaps

3. Evaluate Ability to Capture Clinical Evidence
Gathering clinical evidence under the MDR will have a major impact on how device products are designed, tested, and marketed. Most significantly, it will require companies to conduct more clinical trials. This will require a large investment in resources to capture and store more data completely and transparently. Many medical device manufacturers still use legacy systems that limit their ability to keep up with the size, quantity, and complexity of today’s clinical trials, and this challenge is multiplied with the requirements of the MDR.
4. Implementation and Ongoing Compliance
Medical device manufacturers will have to consider how to allot time and resources to support MDR. Securing executive management support is essential to the success of the program. New educational initiatives can help management comprehend the MDR’s magnitude of change and impact on the business. Beyond the initial implementation phase, manufacturers will need to establish ongoing compliance activities to support the more stringent requirements around clinical evaluation, quality management, and post-market surveillance data management.
A Catalyst for Change
Even as device manufacturers bring remarkable innovations to market, they have largely relied on a combination of manual, paper-based processes and on-premise legacy systems with email for information sharing. These existing systems and processes will struggle in the post-MDR world. Consequently, the MDR may be the catalyst that prompts forward-thinking companies to improve their systems and adopt modern technology.
In many ways, the device landscape mirrors the experience of pharmaceutical companies five to 10 years ago. At that time, changing market conditions led to dramatic transformation across the industry. “Just as pharma realized when they updated enterprise technology, the device industry can benefit from new cloud platforms that allow companies to manage more with less,” added Tummon.
The cloud enables more transparent document and data management across all functions. Silos between departments crumble, increasing visibility into workflows and document lifecycles. In addition, real-time communication and collaboration with internal and external stakeholders, including regulatory authorities, improves dramatically.
Modern systems make regulatory compliance easier to achieve and maintain. Tasks are automatically documented throughout the entire product lifecycle, establishing an audit trail from the moment a record enters a system until archival. With built-in metrics, companies can generate reports for both internal and external use and capture valuable insights on performance.
“Advanced technology becomes critical as we are forced to conduct more clinical studies,” Tummon added. “It’s not sustainable to hire more and more people to manage increased numbers of studies. At Integra, we want to completely eliminate paper and paper-based processes, as well as automate and generate reports at the press of a button. With streamlined processes and capabilities to complete tasks in real time, from anywhere, we can optimize staff time and improve execution.”
The MDR promises to disrupt existing processes for device manufacturers and distributors globally. It will add a new layer of compliance requirements and force more clinical testing of existing and new products. Achieving compliance requires proper preparation, but it will still be difficult—even more so with outdated technology. Adopting new solutions that are flexible, transparent, and scalable will not only help ensure MDR compliance, but also improve overall efficiency and innovation that is crucial to success in any regulatory environment.
References
1 Emergo Outlook Survey 2018 (February 2018).
2 Emergo Blog (October 10, 2018) “Medical Device Companies Underprepared for MDR,” by Stewart Eisenhart.
Melonie Warfel has more than 25 years of technology experience, with deep expertise across multiple verticals—including life sciences, healthcare, manufacturing, and government. At Veeva Systems, Warfel is responsible for global business development and product strategy for the medical devices market, including development and expansion, solution identification, and go-to-market activities. Prior to joining Veeva, Warfel launched and grew Pegasystems’ Life Sciences Business Unit, leveraging the Pega Platform to identify and develop solutions across R&D and commercial. She joined Pegasystems following 19 years with Adobe Systems, where she drove the development of the company’s vertical business strategy. Warfel can be reached at melonie.warfel@veeva.com.