Dorine Rivers, Ph.D., P.M.P., CEO, Alpha 81 Inc.03.07.17
No matter the medical device or product, no matter how brilliant you know your idea may be, applications of medical devices are specialized, and therefore, the chain of events that bring that product or device to market usually follow a well-thought out course of action.
The beginning of such an adventure—and it is just that, an adventure—may spawn from an event a practitioner experiences in the operating room. Or it may appear as a plausible solution to a problem while working in the office. It could come from conversations with peers and associates. Or it may crawl out of his or her superior temporal gyrus at 3 a.m. and sound like this:
“Yeah, I’ve got a really awesome idea for a really cool device that will change the medical industry forever and I‘m going to invent it and save the world! Oh…and get really rich, too!”
Maybe or maybe not.
Why not?
Because recognizing and developing well-targeted, innovative products and bringing them to market on schedule and within budget is complicated. You may have a really cool invention, but do you know how to get it into the hands of others so they can benefit from your brilliance?
Maybe or maybe not.
The processes and systems of value that take place from initial concept to device development, and ultimately the end use of that device, follows a path that is both complex and difficult to visualize and put into action. Launching a medical device out of your head and into the operating room or office, or onto the shelf “looks easy, does hard.”
As you dive into what it takes to make it happen and learn about things you didn’t know existed, a number of questions keep you up at night:
This is where knowing that you don’t know what you don’t know comes in handy. The next step? You bring experts on board to help you navigate through the process. After all, your goal is not just to create your masterpiece, but to have others actually use your device and also to monetize your great idea.
What’s Involved in the Development Process
Several key components are involved in the stages and processes of any new medical device concept. The simplified path for the development of that concept is: Concept, Research, Development, Regulation, and Marketing.
To quote Ron Popeil of Ronco fame, “But wait, there’s more!”
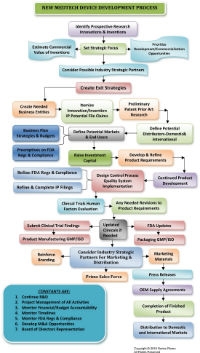
When you peel back the endless layers of the “I’ve got a killer idea” thought, things you need to know (and things you don’t know you need to know) begin to unravel. Soon, the process is far more complicated than you realized (see the flow chart; click it to view a larger version).
Whether used to create a new innovation, redesign an existing device, or evaluate critical paths and bottlenecks, the successful completion of the complexities of this entire process will help to ensure the timely introduction of new medical technologies (i.e., your innovative device).
Few companies, if any, are able to fulfill all of the needed processes and systems in house. Outsourcing needed areas for product development ensures not only the probability of working with competent individuals for that particular process, but eliminates mistakes one might make in trying to save money by doing things not even a video on YouTube could teach.
When seeking a product development company to get you through the labyrinth of a new medical device concept and into the market, that company should possess the capabilities needed to complete the following processes and systems.
1. Identify Worthy Ideas, Innovations, and Inventions
It’s important to have an understanding of the differences between creativity, invention, and innovation. Creativity is the ability to come up with new ideas or concepts. It has nothing to do with value or monetizing the idea. Innovation is the process where value is created in the form of a new solution to a problem, and occurs when a need is identified and a product is developed to meet that need. Innovators use existing technology in new ways. Invention, on the other hand, involves a filed patent. It results in a better mousetrap and new way of doing something, and is an improvement or new idea that must be proven to be different and often superior. Paramount to moving the idea, innovation, or invention toward success is remembering that it will not be worth much if nobody wants to buy it. Identifying consumer wants and needs, and subsequently developing the product or service to meet them, is an initial step that must be completed before moving onto other steps in the process.
2. Patent Research, Intellectual Property, and Patent Development
This includes researching existing and/or potential competition, as well as patent defense mitigation. The protection of intellectual property (IP) is essential to your project. In return, it stimulates medical advances, economic growth, and the introduction of new medical devices. You will be better positioned for compensation for your contribution to the field when the necessary and cautionary steps are taken to secure the concept. Unless you are a knowledgeable patent attorney that is able to complete these tasks, it is advisable to find one to advise and help legally and properly bring the concept to fruition. A reputable project developer will have worked with competent patent attorneys and can suggest one if so desired.
3. Develop Business Formations
What business name and what type of business entity will you select for your project? Entity options may include a C Corporation, S Corporation, or Limited Liability Corporation (LLC). Both legal and tax advice will help you decide which entity will serve you best.
4. Strategic Alliances for Investment Capital
Finding money is almost always one of the first problems that arises. Many investment avenues are available including, but not limited to, angel, private placement, venture capital, bridge, initial secondary public offerings, and family and friends. As in all other aspects of this process, a knowledgeable, competent investment advisor may save you time, money, and even the viability of your project.
5. Asset Growth
Create asset growth through identification, development, and execution of innovative technology-based products via business plans and strategies, and then continually monitor progress.
6. Oversight of Financials
Implement and oversee financial activities, including budgets, accounting systems, and financial reporting. Engaging competent advisors, conducting periodic reviews of the financial status of your company, and maintaining proper records will aid you in presenting your company accurately and completely each step of the way.
7. FDA Registration, Regulatory, and Compliance
This segment of the process will end your project quickly if you do not dot all of your i’s and cross all of your t’s. It is ongoing throughout your device development and is an intricate part of every step you take. The first item the FDA looks for in a submission is how the product was developed. Did the developer follow a formal Design Control Process and do they have evidence of compliance with the process? Generally, design controls for medical devices are part of a broader Quality Management System (QMS) for the developing company.
When a U.S. Food and Drug Administration (FDA) investigator reviews your design control requirements, they will evaluate the control process, not determine if the design is appropriate, safe, or effective. The challenges of incorporating the design and development process into your QMS can be daunting. Since a significant number of medical device recalls are due to design problems, it is advisable for design controls to be in place prior to approval of the system-level requirements document and after completion of the feasibility phase.
For more information, review the Code of Federal Regulations 21 CFR 820.30: http://bit.ly/fda21
The international industry standard defining the QMS is ISO 13485, which outlines all the various procedures and processes a well-run company needs to produce a quality, high reliability product.
A formal submission to the FDA is required to receive clearance to market and sell medical products in the United States. This submission has a wide range of documents that are a natural product of the QMS and design control process, including the product development plan, formal product requirement specifications, software requirements specification, hardware requirements specification, and verification test procedures and reports. These are the primary elements of an FDA submission from a design standpoint.
8. Clinical Trials/Studies Project Management, Execution, and Compliance
Managing clinical trials of any size and complexity requires an expert in this particular field. This portion of the device development process will have five inherent process stages: initiating, planning, executing, monitoring and controlling, and analysis and reporting.
Select a trial manager who knows how to include the details of the arrangements for developing and monitoring all aspects of the clinical trial, and also possesses the communication skills to keep you up to date.
To select a trial manager that will properly serve you and the specific clinical trial your device or product requires, take care to discover the following attributes.
The market for medical devices is not a single channel, but rather a plethora of buyers in various audiences, such as doctors, hospitals, clinics, and consumers. Regardless of the product, it is imperative to get doctors on board with the device. If the medical professionals in your intended marketing space don’t believe the device is effective and/or it is too difficult to use, they won’t use it. And word will spread.
It’s also important to implement a consumer campaign because consumers are going to investigate what procedure is right for them, and then ask their doctor about it. If you are lucky enough to find a marketing partner who is well-versed in digital marketing and knowledgeable about virtual product tours, you may benefit from their advanced expertise.
10. Design for Manufacturability
Paramount to a successful end product is the importance of your device engineer working closely with the manufacturer in the design phase. This collaboration builds value into the product. For example, if design changes can be made to increase production efficiency while meeting the end-use requirements, it’s a win-win for all involved. At the end of the day, the product needs to be able to be manufactured in a cost-effective manner, so design with the end in mind. Some of the many additional considerations in this development stage include the enterprise license, reproduction, territory, term, termination rights, fees, warranty and disclaimer, infringement indemnity, limitation of liability, assignability, and miscellaneous contract provisions. It’s a significant number of considerations to juggle so think expert, and then secure one.
11. Thinking Ahead
Transactional activities—business sales, mergers, acquisitions, due diligence, negotiations, and implementation—should initially be considered at the beginning of the process in terms of a potential exit strategy, and then revisited throughout the product development process. Sometimes, corporate partnering or strategic alliances are formed at this juncture in order to move the project ahead more efficiently. By way of definition, corporate partnering is not the same as a strategic alliance, in that a strategic alliance may be shorter in duration, less formal, and/or contain fewer contractual or transactional elements. Your business sales intermediary, often a financial advisor, should possess the knowledge and skills needed to execute these activities on your behalf.
12. Taking the Lead
Leadership throughout the development process, including research and development, executive and general management of operations, intellectual property, regulatory affairs, quality assurance, process engineering, and domestic and international manufacturing operations for GMP/ISO medical device manufacturers are some of the qualities needed to keep the process afloat. The project developer and project managers will assist in this complexity of organizational and management skills, leaving you time to think of your next great idea.
Planning and Playing to Win
Awareness of what needs to get done is the first big step in getting a medical device from concept to completion. This is where experts are brought on board to successfully navigate the process. After all, the goal is not just to create a masterpiece; the goal is to have others actually use the device and monetize a brilliant concept.
Spend significant time at the front end of the process discovering what the marketplace needs, rather than creating a glossy marketing campaign to sell something no one may want. Next, find experts to assist in each process phase. Last, but certainly not least, keep your arms and legs in at all times and enjoy the ride.
Dr. Dorine Rivers holds a Ph.D. in business management, specializing in human and organizational systems, and a Project Management Professional (PMP) certification. With more than 30 years of experience, she specializes in business and product development for existing companies and startups. Dr. Rivers has been published globally in various newspapers, business magazines, and trade journals. She is the CEO of Alpha 81 Inc., a business and product development corporation, where ideas become things. Her clients range from startups to global companies seeking new product development expertise. She can be contacted at Rivers@Alpha81.com.
The beginning of such an adventure—and it is just that, an adventure—may spawn from an event a practitioner experiences in the operating room. Or it may appear as a plausible solution to a problem while working in the office. It could come from conversations with peers and associates. Or it may crawl out of his or her superior temporal gyrus at 3 a.m. and sound like this:
“Yeah, I’ve got a really awesome idea for a really cool device that will change the medical industry forever and I‘m going to invent it and save the world! Oh…and get really rich, too!”
Maybe or maybe not.
Why not?
Because recognizing and developing well-targeted, innovative products and bringing them to market on schedule and within budget is complicated. You may have a really cool invention, but do you know how to get it into the hands of others so they can benefit from your brilliance?
Maybe or maybe not.
The processes and systems of value that take place from initial concept to device development, and ultimately the end use of that device, follows a path that is both complex and difficult to visualize and put into action. Launching a medical device out of your head and into the operating room or office, or onto the shelf “looks easy, does hard.”
As you dive into what it takes to make it happen and learn about things you didn’t know existed, a number of questions keep you up at night:
- How do I know someone isn’t already secretly working on this and will beat me to the market?
- I know my idea is worth billions of dollars. How do I convince investors? Where do I find an investor who will recognize the genius of it all?
- What is a premarket notification 510(k) submission form? Where do I get one? How do I fill this thing out?
- What type of device studies do the IDE regulations (21 CFR part 812) cover? What do those numbers mean?
- Do I need a strategic partner to help market my device and where do I find one? For that matter, how do you define “strategic”?
- How do I get peer-to-peer endorsements? I just figured out that rejection can make or break the success of my product or device. If my device is difficult to use, or if medical professionals don’t believe in the efficacy of the device or product, it won’t get used, and ultimately, they will stop purchasing it. What do I do?
- My idea is so awesome that a company will want to buy my company right now. What is it worth? How do I do this so I can get on with my life on the beaches of Costa Rica?
This is where knowing that you don’t know what you don’t know comes in handy. The next step? You bring experts on board to help you navigate through the process. After all, your goal is not just to create your masterpiece, but to have others actually use your device and also to monetize your great idea.
What’s Involved in the Development Process
Several key components are involved in the stages and processes of any new medical device concept. The simplified path for the development of that concept is: Concept, Research, Development, Regulation, and Marketing.
To quote Ron Popeil of Ronco fame, “But wait, there’s more!”
When you peel back the endless layers of the “I’ve got a killer idea” thought, things you need to know (and things you don’t know you need to know) begin to unravel. Soon, the process is far more complicated than you realized (see the flow chart; click it to view a larger version).
Whether used to create a new innovation, redesign an existing device, or evaluate critical paths and bottlenecks, the successful completion of the complexities of this entire process will help to ensure the timely introduction of new medical technologies (i.e., your innovative device).
Few companies, if any, are able to fulfill all of the needed processes and systems in house. Outsourcing needed areas for product development ensures not only the probability of working with competent individuals for that particular process, but eliminates mistakes one might make in trying to save money by doing things not even a video on YouTube could teach.
When seeking a product development company to get you through the labyrinth of a new medical device concept and into the market, that company should possess the capabilities needed to complete the following processes and systems.
1. Identify Worthy Ideas, Innovations, and Inventions
It’s important to have an understanding of the differences between creativity, invention, and innovation. Creativity is the ability to come up with new ideas or concepts. It has nothing to do with value or monetizing the idea. Innovation is the process where value is created in the form of a new solution to a problem, and occurs when a need is identified and a product is developed to meet that need. Innovators use existing technology in new ways. Invention, on the other hand, involves a filed patent. It results in a better mousetrap and new way of doing something, and is an improvement or new idea that must be proven to be different and often superior. Paramount to moving the idea, innovation, or invention toward success is remembering that it will not be worth much if nobody wants to buy it. Identifying consumer wants and needs, and subsequently developing the product or service to meet them, is an initial step that must be completed before moving onto other steps in the process.
2. Patent Research, Intellectual Property, and Patent Development
This includes researching existing and/or potential competition, as well as patent defense mitigation. The protection of intellectual property (IP) is essential to your project. In return, it stimulates medical advances, economic growth, and the introduction of new medical devices. You will be better positioned for compensation for your contribution to the field when the necessary and cautionary steps are taken to secure the concept. Unless you are a knowledgeable patent attorney that is able to complete these tasks, it is advisable to find one to advise and help legally and properly bring the concept to fruition. A reputable project developer will have worked with competent patent attorneys and can suggest one if so desired.
3. Develop Business Formations
What business name and what type of business entity will you select for your project? Entity options may include a C Corporation, S Corporation, or Limited Liability Corporation (LLC). Both legal and tax advice will help you decide which entity will serve you best.
4. Strategic Alliances for Investment Capital
Finding money is almost always one of the first problems that arises. Many investment avenues are available including, but not limited to, angel, private placement, venture capital, bridge, initial secondary public offerings, and family and friends. As in all other aspects of this process, a knowledgeable, competent investment advisor may save you time, money, and even the viability of your project.
5. Asset Growth
Create asset growth through identification, development, and execution of innovative technology-based products via business plans and strategies, and then continually monitor progress.
6. Oversight of Financials
Implement and oversee financial activities, including budgets, accounting systems, and financial reporting. Engaging competent advisors, conducting periodic reviews of the financial status of your company, and maintaining proper records will aid you in presenting your company accurately and completely each step of the way.
7. FDA Registration, Regulatory, and Compliance
This segment of the process will end your project quickly if you do not dot all of your i’s and cross all of your t’s. It is ongoing throughout your device development and is an intricate part of every step you take. The first item the FDA looks for in a submission is how the product was developed. Did the developer follow a formal Design Control Process and do they have evidence of compliance with the process? Generally, design controls for medical devices are part of a broader Quality Management System (QMS) for the developing company.
When a U.S. Food and Drug Administration (FDA) investigator reviews your design control requirements, they will evaluate the control process, not determine if the design is appropriate, safe, or effective. The challenges of incorporating the design and development process into your QMS can be daunting. Since a significant number of medical device recalls are due to design problems, it is advisable for design controls to be in place prior to approval of the system-level requirements document and after completion of the feasibility phase.
For more information, review the Code of Federal Regulations 21 CFR 820.30: http://bit.ly/fda21
The international industry standard defining the QMS is ISO 13485, which outlines all the various procedures and processes a well-run company needs to produce a quality, high reliability product.
A formal submission to the FDA is required to receive clearance to market and sell medical products in the United States. This submission has a wide range of documents that are a natural product of the QMS and design control process, including the product development plan, formal product requirement specifications, software requirements specification, hardware requirements specification, and verification test procedures and reports. These are the primary elements of an FDA submission from a design standpoint.
8. Clinical Trials/Studies Project Management, Execution, and Compliance
Managing clinical trials of any size and complexity requires an expert in this particular field. This portion of the device development process will have five inherent process stages: initiating, planning, executing, monitoring and controlling, and analysis and reporting.
Select a trial manager who knows how to include the details of the arrangements for developing and monitoring all aspects of the clinical trial, and also possesses the communication skills to keep you up to date.
To select a trial manager that will properly serve you and the specific clinical trial your device or product requires, take care to discover the following attributes.
- Qualify the contract research organization (CRO) by visiting the facility and checking out the range of expertise within the CRO. Hold meetings with management, scientists, and quality assurance personnel who would be involved in your trial.
- Ensure the proposed CRO and selected professionals within that company are capable of doing the kind of work required (device vs. product vs. procedure) and they work well as a team.
- Conduct an investigator meeting where all investigators and site staff are informed of the particularities of your clinical trial. During this meeting, be sure to provide background information for the clinical trial, why it is being conducted, and its clinical endpoints. This meeting is interactive in nature, and gives the participants a chance to ask questions.
- Since the clinical trial must be supervised by a qualified person, confirm qualifications specific to your applicable regulations and guidelines.
The market for medical devices is not a single channel, but rather a plethora of buyers in various audiences, such as doctors, hospitals, clinics, and consumers. Regardless of the product, it is imperative to get doctors on board with the device. If the medical professionals in your intended marketing space don’t believe the device is effective and/or it is too difficult to use, they won’t use it. And word will spread.
It’s also important to implement a consumer campaign because consumers are going to investigate what procedure is right for them, and then ask their doctor about it. If you are lucky enough to find a marketing partner who is well-versed in digital marketing and knowledgeable about virtual product tours, you may benefit from their advanced expertise.
10. Design for Manufacturability
Paramount to a successful end product is the importance of your device engineer working closely with the manufacturer in the design phase. This collaboration builds value into the product. For example, if design changes can be made to increase production efficiency while meeting the end-use requirements, it’s a win-win for all involved. At the end of the day, the product needs to be able to be manufactured in a cost-effective manner, so design with the end in mind. Some of the many additional considerations in this development stage include the enterprise license, reproduction, territory, term, termination rights, fees, warranty and disclaimer, infringement indemnity, limitation of liability, assignability, and miscellaneous contract provisions. It’s a significant number of considerations to juggle so think expert, and then secure one.
11. Thinking Ahead
Transactional activities—business sales, mergers, acquisitions, due diligence, negotiations, and implementation—should initially be considered at the beginning of the process in terms of a potential exit strategy, and then revisited throughout the product development process. Sometimes, corporate partnering or strategic alliances are formed at this juncture in order to move the project ahead more efficiently. By way of definition, corporate partnering is not the same as a strategic alliance, in that a strategic alliance may be shorter in duration, less formal, and/or contain fewer contractual or transactional elements. Your business sales intermediary, often a financial advisor, should possess the knowledge and skills needed to execute these activities on your behalf.
12. Taking the Lead
Leadership throughout the development process, including research and development, executive and general management of operations, intellectual property, regulatory affairs, quality assurance, process engineering, and domestic and international manufacturing operations for GMP/ISO medical device manufacturers are some of the qualities needed to keep the process afloat. The project developer and project managers will assist in this complexity of organizational and management skills, leaving you time to think of your next great idea.
Planning and Playing to Win
Awareness of what needs to get done is the first big step in getting a medical device from concept to completion. This is where experts are brought on board to successfully navigate the process. After all, the goal is not just to create a masterpiece; the goal is to have others actually use the device and monetize a brilliant concept.
Spend significant time at the front end of the process discovering what the marketplace needs, rather than creating a glossy marketing campaign to sell something no one may want. Next, find experts to assist in each process phase. Last, but certainly not least, keep your arms and legs in at all times and enjoy the ride.
Dr. Dorine Rivers holds a Ph.D. in business management, specializing in human and organizational systems, and a Project Management Professional (PMP) certification. With more than 30 years of experience, she specializes in business and product development for existing companies and startups. Dr. Rivers has been published globally in various newspapers, business magazines, and trade journals. She is the CEO of Alpha 81 Inc., a business and product development corporation, where ideas become things. Her clients range from startups to global companies seeking new product development expertise. She can be contacted at Rivers@Alpha81.com.