Steve Maylish, Chief Commercial Officer, Fusion Biotec, and Luke Helm, Director of Business Development, Symbient Product Development10.12.16
When considering complex medical instrument development, a specialized design team familiar with the device’s field and led by a system expert is critical to success. If the design requires creative problem-solving and new technology, experts with domain experience can be extremely helpful. Even though experts reside internally for many established companies, there are still challenges. Specialized engineering firms offer a great alternative, provided they have applicable expertise and seasoned teams. In either case, a successful team includes creativity, industry experience, domain expertise, and a system expert.
In the beginning of a project, there should be an understanding of its complexity and technical risks. This often sets the stage for a proof-of-concept or feasibility system driven by the engineering or research teams. The early feasibility phase often proves the viability of the idea; an effective design team will retire risk early and often. Consider an electrochemical sensor being used for diagnostics: Early sensor fabrication and testing are essential to project success. When the concept is considered feasible, formal development can begin.
But what if a project is more complex? The development team should reflect this reality, like a football team built of specialized players. A quarterback doesn’t send his center out for a 50-yard pass, and a project manager shouldn’t send his mechanical engineer out for a quick course in microfluidics. Even though domain experts are more expensive than employees, they contribute their expertise only when needed, like a reliable kicker sustaining the team’s momentum. To continue the analogy—winning football teams are led by great quarterbacks; a successful design team is led by a great project manager or system engineer.
Specialized design teams working together for years notice and solve common technical challenges every day. These teams mesh well together and understand how to design a commercial instrument. MVPs (minimal viable products) provide the “good enough” solution needed for the marketplace, and follow-on software revisions provide additional features and refresh systems as they age. Designing robust and maintainable software is key to a product’s longevity.
The chart below shows some of the expertise needed for complex diagnostic instrument development. Specialized expertise is crucial to success—project teams should closely examine these functions, because each project is different.
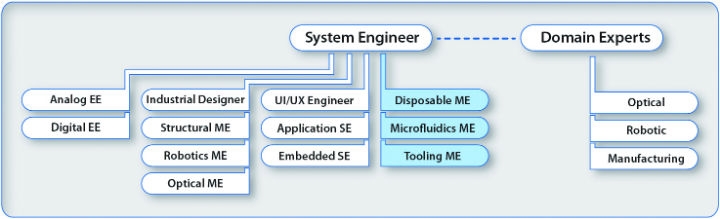
There are two main areas in any design and development effort—creating and implementing. Some engineers cover both areas, but others fall into one of the two categories. Many creative design engineers aren’t excited about documentation, and staff engineers on existing product lines usually lack the creativity and breadth of experience needed to develop new designs. Therefore, it’s important to make sure both areas are represented on the team.
The saying goes, “If you give an instrument to a team of engineers, some will want to take it apart and see how it works, and some will want to box it up and ship it out.” However, implementers are more than engineers completing documentation. They are responsible for correctly defining the instrument, adapting it when necessary, amending specifications, integrating the final system, and testing, verifying, and preparing it for U.S. Food and Drug Administration submission. They are also responsible for helping transfer to manufacturing.
Any engineering discipline can also be separated by expertise; for example, analog and digital electrical engineering, disposable and structural mechanical engineering, and application and embedded software engineering. For complex instrumentation, this bifurcation can be just the beginning of defining the expertise needed for the design team. Optics, robotics, or microfluidics can add another dimension to a team requiring seven-foot high jumpers, where no five-footers will do.
In MPO’s June issue, this column took a deep dive into software engineering. Consider disposable mechanical design—specifically microfluidics and cartridges, for example—translating an assay from the lab into a disposable cartridge. In order to automate this process, a protocol should be well defined. The first stage is identifying and describing in detail the actual steps that a lab technician takes to complete the assay. For example, “perform PCR” entails multiple steps that must be described in detail, such as the order of operation, volumes of sample and reagents, mixing characterization, heating time, and temperature cycling. Assay developers should also test acceptable tolerances for these steps. Wider tolerances provide fewer constraints in the design, lowering development and manufacturing costs. Untested or unnecessarily tight tolerances increase complexity and cost.
Once requirements and protocols are well defined, disposable mechanical design can begin. Here, experience should inform design and provide several options. Engineers with extensive experience in microfluidic disposable development will have “off-the-shelf” solutions for common processes such as sample collecting, valving, metering, mixing, sealing, filtering, pumping, storing reagents, etc. These solutions should be available as non-proprietary or licensed intellectual property and be re-configurable for the specific applications.
The feasibility, manufacturability, technical risk, cost-per-unit, and overall performance should be evaluated by a team of experts. The team should not only develop creative ideas, but assess tradeoffs, prioritize, and communicate the ideas. These tradeoffs often include features, performance, cost, and complexity with the disposable and the instrument. One common way of meeting disposable cost targets is to transfer cartridge functions to the instrument. Adding functions to the cartridge increases its complexity, part count, and assembly steps—the same functions may be more feasible in the instrument. On the other hand, certain active functions in the instrument can create sterilization and cross-contamination challenges not seen in a disposable cartridge. Cartridge costs often take priority, as there are cost constraints on tests and their sales drive revenue models.
Disposables and microfluidics have unique development challenges: material selection, micromachining, molding, laser cutting, on-board reservoirs, aseptic filling, and sterilization, just to name a few. The rapid prototyping process can also be uniquely challenging. It typically begins with a combination of stereolithography, machined parts, and off-the-shelf components. Effective microfluidic designers look to minimize “discovery” work and keep iterations to a minimum by utilizing their experience with prior successful designs. If any cartridge functions are anticipated to require multiple iterations, they should be prototyped separately to reduce variables. Once these functions are working well, an integrated prototype should then be created and tested.
Rapid prototyping methods help finalize much of the cartridge. However, in most cases, actual injection-molded parts must be developed and assembled using prototype tooling. Examples include reproducing high-quality microfluidic channels, achieving the optical clarity needed for detection, and finalizing plastic assembly methods. In certain cases, fabricating tools and testing prototypes using injection-molded parts are the only ways to verify the design and replicate final manufacturing processes.
This requires extensive experience in design-for-manufacturing, tool design, and assembly to ensure scalability. Implemented correctly, injection-molded prototypes will not only improve performance over rapid prototypes, but enable extensive testing, characterization, and design refinement before committing to production tooling. As compared to rapid prototypes, final materials can be used, parts strengthened, walls made thinner, tighter tolerances held, and overall quality improved. Statistically significant quantities of parts and assemblies should be fabricated and tested, developing confidence in the design, assembly process, and necessary refinements.
When developing microfluidic disposables, it’s crucial to use very high-quality prototype tooling from shops that offer specialty services. These shops should have a unique combination of expertise in complex, tight tolerance, fine feature CNC machining, and prototype injection molding. Less-than-optimal quality of the tool can be confused with a necessary design change—for that reason, rather than prototype molding machines, production-grade injection molding equipment should be employed. The closer fabrication methods are to actual manufacturing processes, the more productive the prototypes and development process will be.
Molded prototypes also enable assembly steps to be fully developed and tested. Assembly methods such as ultrasonic welding, laser welding, heat sealing, UV bonding, etc., need to be tested and proven on final materials and processes. Any necessary custom assembly fixtures should be developed at this stage as well. This provides a complete package of tooling, processes, and information, which can be transferred to manufacturing to ensure a smooth scale-up to higher volumes.
One common development challenge is the sealing of microfluidic channels in a cartridge. Channel size, landing area between channels, and sealing methods that are compatible with a device’s chemistries must be considered. Another production challenge is the avoidance of bubbles, especially if the detection method is sensitive to them. Bubbles can be created when a sample is put into a cartridge, mixed, transferred, or heated. Teams must design a system that mitigates bubble creation or includes bubble traps before the sample enters the detection area. Seals and seal compression can also present challenges; often, these can be resolved using integrated gaskets with overmolded silicone.
A common challenge with certain detection methods is alignment of the cartridge in the instrument. Features should be designed for automatic orientation and alignment of the cartridge. Some detection methods require ultra-precise alignment—in these cases, special attention should be paid to plastic shrink rates (following molding) and achievable injection molding tolerances of the alignment features designed into the cartridge.
Assembly methods, instrument integration, and detection methods—among other factors—are all affected by the challenges of injection molding. Designing for assembly and planning for scale-up must be balanced with repeatability. Reducing manufacturing costs with multi-cavity tooling can require another layer of planning and expertise to ensure these tolerances can be met. Tolerance stack- up—including factors such as CNC tool machining, multi-cavity molding tolerances, plastic shrink rates, and assembly processes—must all be considered. As parts become larger, they tend to warp, which can be challenging when flatness is a critical dimension.
Laminate structures, including laser-cut and die-cut layers, may be integrated with injection-molded structures. The assembly method, sealing, and integration of these two manufacturing methods must be planned and executed by engineers with significant expertise in these processes. Each method has advantages and limitations, which should be understood and planned for in advance.
Throughout cartridge development, testing and design iteration are essential. Initially, testing is performed using manual methods such as syringe pumps, off-the-shelf valves, pressure-sensitive adhesives, or clamps and gaskets to assemble the parts. As development proceeds, testing must replicate the instrument processes, including mechanical actuators, pneumatics, valves, pumps, and eventually, the detection method applicable to the assay. Engineers will often design and build a prototype test fixture, complete with a nest for the cartridge, in order to prove out performance of the disposable before turning it over to the instrument team. However, collaboration between the instrument and disposable teams should start during rapid prototyping.
For complex medical instrument development, a specialized design team can be invaluable. Domain experts can provide expertise as needed and resolve particular design challenges. Specialized teams know how to design and build commercial instruments, providing innovative and patentable solutions along the way.
Steve Maylish has been part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences.
Luke Helm is director of business development at Symbient Product Development, a world leader in design engineering of medical and life science devices that use plastics. A 16-year veteran of disruptive technology businesses, he has helped hundreds of scientists and other decision-makers navigate the device development process. He brings a practical perspective and valuable insights that can be applied to avoid pitfalls and encourage a successful product development effort. Luke has a B.S. in business administration and has a background in formal project management.
In the beginning of a project, there should be an understanding of its complexity and technical risks. This often sets the stage for a proof-of-concept or feasibility system driven by the engineering or research teams. The early feasibility phase often proves the viability of the idea; an effective design team will retire risk early and often. Consider an electrochemical sensor being used for diagnostics: Early sensor fabrication and testing are essential to project success. When the concept is considered feasible, formal development can begin.
But what if a project is more complex? The development team should reflect this reality, like a football team built of specialized players. A quarterback doesn’t send his center out for a 50-yard pass, and a project manager shouldn’t send his mechanical engineer out for a quick course in microfluidics. Even though domain experts are more expensive than employees, they contribute their expertise only when needed, like a reliable kicker sustaining the team’s momentum. To continue the analogy—winning football teams are led by great quarterbacks; a successful design team is led by a great project manager or system engineer.
Specialized design teams working together for years notice and solve common technical challenges every day. These teams mesh well together and understand how to design a commercial instrument. MVPs (minimal viable products) provide the “good enough” solution needed for the marketplace, and follow-on software revisions provide additional features and refresh systems as they age. Designing robust and maintainable software is key to a product’s longevity.
The chart below shows some of the expertise needed for complex diagnostic instrument development. Specialized expertise is crucial to success—project teams should closely examine these functions, because each project is different.

There are two main areas in any design and development effort—creating and implementing. Some engineers cover both areas, but others fall into one of the two categories. Many creative design engineers aren’t excited about documentation, and staff engineers on existing product lines usually lack the creativity and breadth of experience needed to develop new designs. Therefore, it’s important to make sure both areas are represented on the team.
The saying goes, “If you give an instrument to a team of engineers, some will want to take it apart and see how it works, and some will want to box it up and ship it out.” However, implementers are more than engineers completing documentation. They are responsible for correctly defining the instrument, adapting it when necessary, amending specifications, integrating the final system, and testing, verifying, and preparing it for U.S. Food and Drug Administration submission. They are also responsible for helping transfer to manufacturing.
Any engineering discipline can also be separated by expertise; for example, analog and digital electrical engineering, disposable and structural mechanical engineering, and application and embedded software engineering. For complex instrumentation, this bifurcation can be just the beginning of defining the expertise needed for the design team. Optics, robotics, or microfluidics can add another dimension to a team requiring seven-foot high jumpers, where no five-footers will do.
In MPO’s June issue, this column took a deep dive into software engineering. Consider disposable mechanical design—specifically microfluidics and cartridges, for example—translating an assay from the lab into a disposable cartridge. In order to automate this process, a protocol should be well defined. The first stage is identifying and describing in detail the actual steps that a lab technician takes to complete the assay. For example, “perform PCR” entails multiple steps that must be described in detail, such as the order of operation, volumes of sample and reagents, mixing characterization, heating time, and temperature cycling. Assay developers should also test acceptable tolerances for these steps. Wider tolerances provide fewer constraints in the design, lowering development and manufacturing costs. Untested or unnecessarily tight tolerances increase complexity and cost.
Once requirements and protocols are well defined, disposable mechanical design can begin. Here, experience should inform design and provide several options. Engineers with extensive experience in microfluidic disposable development will have “off-the-shelf” solutions for common processes such as sample collecting, valving, metering, mixing, sealing, filtering, pumping, storing reagents, etc. These solutions should be available as non-proprietary or licensed intellectual property and be re-configurable for the specific applications.
The feasibility, manufacturability, technical risk, cost-per-unit, and overall performance should be evaluated by a team of experts. The team should not only develop creative ideas, but assess tradeoffs, prioritize, and communicate the ideas. These tradeoffs often include features, performance, cost, and complexity with the disposable and the instrument. One common way of meeting disposable cost targets is to transfer cartridge functions to the instrument. Adding functions to the cartridge increases its complexity, part count, and assembly steps—the same functions may be more feasible in the instrument. On the other hand, certain active functions in the instrument can create sterilization and cross-contamination challenges not seen in a disposable cartridge. Cartridge costs often take priority, as there are cost constraints on tests and their sales drive revenue models.
Disposables and microfluidics have unique development challenges: material selection, micromachining, molding, laser cutting, on-board reservoirs, aseptic filling, and sterilization, just to name a few. The rapid prototyping process can also be uniquely challenging. It typically begins with a combination of stereolithography, machined parts, and off-the-shelf components. Effective microfluidic designers look to minimize “discovery” work and keep iterations to a minimum by utilizing their experience with prior successful designs. If any cartridge functions are anticipated to require multiple iterations, they should be prototyped separately to reduce variables. Once these functions are working well, an integrated prototype should then be created and tested.
Rapid prototyping methods help finalize much of the cartridge. However, in most cases, actual injection-molded parts must be developed and assembled using prototype tooling. Examples include reproducing high-quality microfluidic channels, achieving the optical clarity needed for detection, and finalizing plastic assembly methods. In certain cases, fabricating tools and testing prototypes using injection-molded parts are the only ways to verify the design and replicate final manufacturing processes.
This requires extensive experience in design-for-manufacturing, tool design, and assembly to ensure scalability. Implemented correctly, injection-molded prototypes will not only improve performance over rapid prototypes, but enable extensive testing, characterization, and design refinement before committing to production tooling. As compared to rapid prototypes, final materials can be used, parts strengthened, walls made thinner, tighter tolerances held, and overall quality improved. Statistically significant quantities of parts and assemblies should be fabricated and tested, developing confidence in the design, assembly process, and necessary refinements.
When developing microfluidic disposables, it’s crucial to use very high-quality prototype tooling from shops that offer specialty services. These shops should have a unique combination of expertise in complex, tight tolerance, fine feature CNC machining, and prototype injection molding. Less-than-optimal quality of the tool can be confused with a necessary design change—for that reason, rather than prototype molding machines, production-grade injection molding equipment should be employed. The closer fabrication methods are to actual manufacturing processes, the more productive the prototypes and development process will be.
Molded prototypes also enable assembly steps to be fully developed and tested. Assembly methods such as ultrasonic welding, laser welding, heat sealing, UV bonding, etc., need to be tested and proven on final materials and processes. Any necessary custom assembly fixtures should be developed at this stage as well. This provides a complete package of tooling, processes, and information, which can be transferred to manufacturing to ensure a smooth scale-up to higher volumes.
One common development challenge is the sealing of microfluidic channels in a cartridge. Channel size, landing area between channels, and sealing methods that are compatible with a device’s chemistries must be considered. Another production challenge is the avoidance of bubbles, especially if the detection method is sensitive to them. Bubbles can be created when a sample is put into a cartridge, mixed, transferred, or heated. Teams must design a system that mitigates bubble creation or includes bubble traps before the sample enters the detection area. Seals and seal compression can also present challenges; often, these can be resolved using integrated gaskets with overmolded silicone.
A common challenge with certain detection methods is alignment of the cartridge in the instrument. Features should be designed for automatic orientation and alignment of the cartridge. Some detection methods require ultra-precise alignment—in these cases, special attention should be paid to plastic shrink rates (following molding) and achievable injection molding tolerances of the alignment features designed into the cartridge.
Assembly methods, instrument integration, and detection methods—among other factors—are all affected by the challenges of injection molding. Designing for assembly and planning for scale-up must be balanced with repeatability. Reducing manufacturing costs with multi-cavity tooling can require another layer of planning and expertise to ensure these tolerances can be met. Tolerance stack- up—including factors such as CNC tool machining, multi-cavity molding tolerances, plastic shrink rates, and assembly processes—must all be considered. As parts become larger, they tend to warp, which can be challenging when flatness is a critical dimension.
Laminate structures, including laser-cut and die-cut layers, may be integrated with injection-molded structures. The assembly method, sealing, and integration of these two manufacturing methods must be planned and executed by engineers with significant expertise in these processes. Each method has advantages and limitations, which should be understood and planned for in advance.
Throughout cartridge development, testing and design iteration are essential. Initially, testing is performed using manual methods such as syringe pumps, off-the-shelf valves, pressure-sensitive adhesives, or clamps and gaskets to assemble the parts. As development proceeds, testing must replicate the instrument processes, including mechanical actuators, pneumatics, valves, pumps, and eventually, the detection method applicable to the assay. Engineers will often design and build a prototype test fixture, complete with a nest for the cartridge, in order to prove out performance of the disposable before turning it over to the instrument team. However, collaboration between the instrument and disposable teams should start during rapid prototyping.
For complex medical instrument development, a specialized design team can be invaluable. Domain experts can provide expertise as needed and resolve particular design challenges. Specialized teams know how to design and build commercial instruments, providing innovative and patentable solutions along the way.
Steve Maylish has been part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences.
Luke Helm is director of business development at Symbient Product Development, a world leader in design engineering of medical and life science devices that use plastics. A 16-year veteran of disruptive technology businesses, he has helped hundreds of scientists and other decision-makers navigate the device development process. He brings a practical perspective and valuable insights that can be applied to avoid pitfalls and encourage a successful product development effort. Luke has a B.S. in business administration and has a background in formal project management.