Steve Maylish and Leanna M. Levine, Fusion Biotec and ALine05.06.16
The demand for rapid diagnostic analysis is putting pressure on companies to move testing out of central laboratories and into the point-of-care environment. According to Markets and Markets Inc.’s latest report, “This market (microfluidics) is mainly driven by the rising demand for point-of-care (POC) testing, increasing demand for microfluidic chip miniaturization as it offers lower testing time and improved portability, growing Asian market, rising incidences of lifestyle diseases, increasing R&D expenditure and healthcare spending, and growing stem cell and cancer research...In 2014, the in-vitro diagnostics application segment accounted for the largest share of the global microfluidics market.”
When testing is closer to the patient, turnaround time, sample touch time, and logistics are all reduced. Rapid results improve patient care and outcomes, and reduce required blood and healthcare resources. Hence, why has it taken so long for POC to be realized?

Steve Maylish is chief commercial officer for Fusion Biotec.
“The microfluidics market is witnessing tremendous growth…However, a complex and time-consuming regulatory approval process, high cost of integrated instruments and microfluidic sensors, and lack of market visibility for microfluidics products are inhibiting the growth of this market to a certain extent. The microfluidics market is expected to reach $7.5 billion by 2020 from $3.1 billion in 2015, at a CAGR of 19.3 percent,” the Markets and Markets report noted.
In large urban areas where extensive infrastructure for blood collection and delivery allows for results in a day or two, a POC test may not make economic sense. Reimbursement codes allow for a certain payment per test, but point-of-service (POS) can also be a factor, as reimbursement typically decreases from central lab to a doctor’s office and home. Tests with POC systems are typically more expensive and often produce results with higher variation when compared to a central lab. Some tests are not appropriate for POC because of the need for sample preparation or for trained lab technicians and laboratory controls.
Yet there are very large market needs for tests in rural or more resource-limited settings where POC systems provide the best option for patient care. In these situations, POC systems have vastly improved by implementing new techniques and technologies. Over the last 10 years, a lot of funding was provided by the Gates Foundation, the U.S. Department of Defense, and the National Institutes of Health to develop POC systems for low-resource settings where infrastructure is limited. These efforts helped focus the field on developing cost-effective solutions suitable for all environments.

Leanna M. Levine is an entrepreneur, technologist, inventor, and founder of ALine.
Keep in mind, however, that POC testing methodologies vary widely in biochemistry, microfluidic technology, and hardware complexity. Ranging from the qualitative lateral flow test, read visually (i.e., pregnancy test), to lab-on-a-chip systems performing multi-step, multiplex assays, varying degrees of precision and accuracy are required. In some cases, a simple screening test is adequate for diagnosis, while in others, results must be known within a range or at a comparative level for accurate diagnosis. On the surface, handheld and tabletop POC diagnostic instruments with microfluidics cartridges can look deceivingly simple. Development of these systems, however, requires an extraordinary design and integration effort, as well as an obsessive focus on system design. In fact, the greater the ease of use, the greater the engineering effort.
With the advent of novel sensors and improved wireless communications, coupled with robust liquid handling strategies and vetted manufacturing processes, POC testing holds vast potential. As the Internet of Things grows, the contribution of more complex POC tests will extend and amplify our ability to analyze both ourselves and our environment. We are at the cusp of an explosion in applications. As we attempt to move diagnostic testing closer to the patient, complex processes will be simplified, optimized, and miniaturized, eventually leading to ubiquitous home and healthcare provider use.
To assemble the right team of experts within one company—combining mechanical, electrical, software, microfluidics, biochemistry, and optics expertise—is risky and not cost effective. Thus, many companies choose to outsource the development, while benchmarking performance of the biochemistry as it integrates with the system.
This is easier said than done. Consider the dynamics of three full-scale development efforts running in parallel and on different schedules, starting at different times, but finishing simultaneously.

As an OEM or startup, how can you stack the cards in your favor? You must be mindful of the critical inflection points in the development process.
The biochemistry or novel sensor system usually starts as a research project, where the diagnostic test is born. As the amount of experimental data mounts, concept viability is established, demonstrating proof-of-concept. Once these experiments are well-characterized, and reproduced, they’re moved out of the research stage and into development. At this point, stakeholders have one thing in mind—how soon can we launch the product in the market? This now requires research be reduced to practice within an instrument with quality control criteria suitable for commercialization. Initially, laboratory controls provide the ability to benchmark the performance of the biochemistry as it’s ported into the instrument and microfluidic system. Later, as the effort matures, quality control will require new protocols that reduce labor intensity, while providing confidence in the results.
At this stage, the microfluidics cartridge developer should be involved. If the test can be done using lateral flow, the fluidics are well understood. Finding a development partner with direct experience in commercializing these tests is important. However, for more complex microfluidic tests that require unique front-end sample prep, or a level of multiplexing and quantitation not possible with lateral flow test strips, an expert in microfluidics is required.
This brings us to a critical inflection point—the timing and ways in which microfluidics are implemented.
To integrate and optimize a variety of dissimilar components coupled together in close proximity is the challenge of microfluidics. It involves a combination of biological and materials science, and engineering to create a robust solution. Numerous process steps can be involved including metering, mixing, liquid dispensing, venting, incubating, deaerating, valving, filtering, sensing, reading, sample prep, lysis, and polymerase chain reaction.
Translating the exact protocol completed in the lab into a microfluidic device can lead to numerous reagent reservoirs and cumbersome mechanical interfaces. A better strategy is to optimize the assay to reduce process steps and number of reagents. This also reduces the cost of developing the microfluidics and making the disposable. In microfluidic systems, the high surface area to volumes, shorter diffusion paths, and smaller thermal masses can rapidly produce a measurement end point. However, if too many reagents are onboard, incorrect washing is implemented, or robust solutions are not employed, the opposite can occur.
Remember, each component of the system that requires separate performance verification is adding complexity and a risk for failure. A focus on optimization here will set the stage for success.
The biochemistry and microfluidics effort must work together in order to find an optimal solution.
If a microfluidics developer has a set of functional solutions that can be reliably incorporated into the disposable, then this greatly increases project success. This is the classic modular approach to development that works so well in hardware. Here, microfluidic functions are broken down into modules, reducing risk and improving functional performance. This allows proven fluidic functions to be combined with custom made sensors (e.g., optical, electrical, etc.), injection molded parts, micro-machined silicon/glass, and more.
Once biochemistry and microfluidics are optimized and the microfluidic cartridge functions identified, we arrive at the second critical inflection point: cartridge transfer.
Microfluidic requirements and the critical interfaces between the instrument and the disposable cartridge must be nailed down. A design freeze at this critical point is not only recommended, but required to keep the project on track.
Just as typical instrument development includes off-the-shelf (OTS) components, such as power supplies and LED displays, so too, should microfluidics. Consider the difference between creating an entirely new product from its component level on up versus integrating OTS components. This analogy is critical on the microfluidics and instrument side, but microfluidics is the gating factor.
Imagine for a minute that your microfluidics developer has few proven solutions for your application or hasn’t combined these functions in the past. Imagine also that once the cartridge is developed, it can’t be tested. It’s now up to the instrument developer to make it work, a task that may be beyond his capabilities.
Instead of using the trial and error learning curve, use existing technology whenever possible. Experienced microfluidic developers should have proven technology solutions and methods for testing and validating them. This should occur before passing the cartridge to the instrument developers, otherwise you might find yourself in a finger-pointing contest.
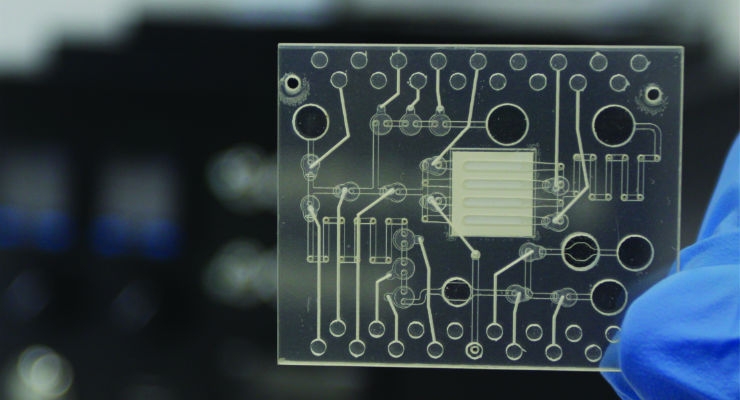
If instrument developers already understand the microfluidic requirements (third image in the above photo slider) and are proficient at incorporating these controls, then critical points in the transfer process are less likely to create problems. Instead of developing ways to solve cartridge inconsistencies, instrument developers can focus on the task at hand.

This leads to a third critical inflection point: system integration.
From an engineering and design-for-manufacturing perspective, the fewer mechanical parts there are and the lower the interconnection tolerances, the better. It is at this point the three development efforts converge to work together. Chemistries may need to be tweaked or modified to run correctly on the cartridge. Cartridge alignment may need to be keyed in the instrument instead of depending on an instrument edge for proper seating. Power requirements for handheld instruments may require better power management and less power consumption.
With thermocycling, the science, engineering, and system design must be coordinated to ensure a design feature in one component doesn’t add complexity or risk to another. Even the nature of the nucleic acid detection test can affect the system design and choice of materials, so it’s prudent to map out reasonable specifications on assay performance at the outset; choose the cartridge design, and interface to the detection and other electro-mechanical components early.
For example, the cartridge material must not react with the chemistry, so inert materials like polypropylene or polycarbonate might be used. The designers must understand how to mold it, attach various layers, best methods for making thermal contact, fluid containment during the thermocycling process, and providing optial clarity for detection.
System integration should be done in stages as sub-systems become available or can be simulated in software. Components for each sub-system should be tested and verified with its control electronics, then integrated into the instrument.
Software should provide a means for communication, early testing, and simulation even if hardware is not available. Biochemistry, microfluidics, and instrument teams should all be able to test their assumptions early and within the actual development process rather than toward the end.
Remember that exact protocols from the lab should not be expected to carry over to cartridge and instrument design. The number of cartridge functions and the precision in features in the cartridge should be minimized in order to keep cost and complexity down and performance up. Revenue models driven by cartridge sales can make or break a startup company if the disposable doesn’t provide sufficient margin.
Microfluidic cartridges need to be tested and verified before being transferred to the instrument developer, preferably with some knowledge of the fluid control components required and an understanding of the actuation routine. Otherwise, the project can be sidetracked with component specifications for the cartridge actuation routine instead of being focused on system integration issues. This can cause significant delays and frustration with trial-and-error processes attempting to solve multiple problems at once. With the right team, there will be a clear path to manufacturing scalability, and cost targets.
Off-the-shelf components in the instrument and proven microfluidic sub-systems should drive the development effort, using as much existing technology as possible. Custom-built modifications to either the cartridge or the instrument should be kept to a minimum.
POC diagnostic development is a complicated effort between multiple teams. The development is complete only when the system works reliably and cost effectively. Biochemisty, microfluidics, and instrument design must collaborate throughout the project to create a commercially successful POC diagnostic system. This is a difficult undertaking to accomplish internally, but very achievable through appropriate outsourcing.
Steve Maylish has been a part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences. After receiving his MBA in 2002, Maylish focused his business development skills on two boutique medical design/manufacturing firms that were acquired. Afterwards, he founded Maytrix Medical to provide business development to third-party engineering firms. Maylish has an A.S. degree in electronics from RETS Electronics Institute, a B.S. in business from the University of La Verne, and an M.B.A. from the University of California, Irvine.
Leanna M. Levine is an entrepreneur, technologist, and inventor. She founded ALine in 2003. Prior to founding ALine, Levine was engaged in new technology development in membrane separations, bioanalysis, and high throughput screening while a member of Monsanto’s corporate R&D. In 1998, she joined Spectrum Laboratories as director of manufacturing and product development. Levine earned her Ph.D. at Washington University, St. Louis (1986), and her bachelor of science degree in biochemistry and bachelor of arts in German from the University of Missouri, Columbia. In 2000, she chaired the Gordon Conference on Bioanalytical Sensors. She has co-authored a dozen publications and also several patents.
When testing is closer to the patient, turnaround time, sample touch time, and logistics are all reduced. Rapid results improve patient care and outcomes, and reduce required blood and healthcare resources. Hence, why has it taken so long for POC to be realized?
Steve Maylish is chief commercial officer for Fusion Biotec.
In large urban areas where extensive infrastructure for blood collection and delivery allows for results in a day or two, a POC test may not make economic sense. Reimbursement codes allow for a certain payment per test, but point-of-service (POS) can also be a factor, as reimbursement typically decreases from central lab to a doctor’s office and home. Tests with POC systems are typically more expensive and often produce results with higher variation when compared to a central lab. Some tests are not appropriate for POC because of the need for sample preparation or for trained lab technicians and laboratory controls.
Yet there are very large market needs for tests in rural or more resource-limited settings where POC systems provide the best option for patient care. In these situations, POC systems have vastly improved by implementing new techniques and technologies. Over the last 10 years, a lot of funding was provided by the Gates Foundation, the U.S. Department of Defense, and the National Institutes of Health to develop POC systems for low-resource settings where infrastructure is limited. These efforts helped focus the field on developing cost-effective solutions suitable for all environments.
Leanna M. Levine is an entrepreneur, technologist, inventor, and founder of ALine.
With the advent of novel sensors and improved wireless communications, coupled with robust liquid handling strategies and vetted manufacturing processes, POC testing holds vast potential. As the Internet of Things grows, the contribution of more complex POC tests will extend and amplify our ability to analyze both ourselves and our environment. We are at the cusp of an explosion in applications. As we attempt to move diagnostic testing closer to the patient, complex processes will be simplified, optimized, and miniaturized, eventually leading to ubiquitous home and healthcare provider use.
To assemble the right team of experts within one company—combining mechanical, electrical, software, microfluidics, biochemistry, and optics expertise—is risky and not cost effective. Thus, many companies choose to outsource the development, while benchmarking performance of the biochemistry as it integrates with the system.
This is easier said than done. Consider the dynamics of three full-scale development efforts running in parallel and on different schedules, starting at different times, but finishing simultaneously.
As an OEM or startup, how can you stack the cards in your favor? You must be mindful of the critical inflection points in the development process.
The biochemistry or novel sensor system usually starts as a research project, where the diagnostic test is born. As the amount of experimental data mounts, concept viability is established, demonstrating proof-of-concept. Once these experiments are well-characterized, and reproduced, they’re moved out of the research stage and into development. At this point, stakeholders have one thing in mind—how soon can we launch the product in the market? This now requires research be reduced to practice within an instrument with quality control criteria suitable for commercialization. Initially, laboratory controls provide the ability to benchmark the performance of the biochemistry as it’s ported into the instrument and microfluidic system. Later, as the effort matures, quality control will require new protocols that reduce labor intensity, while providing confidence in the results.
At this stage, the microfluidics cartridge developer should be involved. If the test can be done using lateral flow, the fluidics are well understood. Finding a development partner with direct experience in commercializing these tests is important. However, for more complex microfluidic tests that require unique front-end sample prep, or a level of multiplexing and quantitation not possible with lateral flow test strips, an expert in microfluidics is required.
This brings us to a critical inflection point—the timing and ways in which microfluidics are implemented.
To integrate and optimize a variety of dissimilar components coupled together in close proximity is the challenge of microfluidics. It involves a combination of biological and materials science, and engineering to create a robust solution. Numerous process steps can be involved including metering, mixing, liquid dispensing, venting, incubating, deaerating, valving, filtering, sensing, reading, sample prep, lysis, and polymerase chain reaction.
Translating the exact protocol completed in the lab into a microfluidic device can lead to numerous reagent reservoirs and cumbersome mechanical interfaces. A better strategy is to optimize the assay to reduce process steps and number of reagents. This also reduces the cost of developing the microfluidics and making the disposable. In microfluidic systems, the high surface area to volumes, shorter diffusion paths, and smaller thermal masses can rapidly produce a measurement end point. However, if too many reagents are onboard, incorrect washing is implemented, or robust solutions are not employed, the opposite can occur.
Remember, each component of the system that requires separate performance verification is adding complexity and a risk for failure. A focus on optimization here will set the stage for success.
The biochemistry and microfluidics effort must work together in order to find an optimal solution.
If a microfluidics developer has a set of functional solutions that can be reliably incorporated into the disposable, then this greatly increases project success. This is the classic modular approach to development that works so well in hardware. Here, microfluidic functions are broken down into modules, reducing risk and improving functional performance. This allows proven fluidic functions to be combined with custom made sensors (e.g., optical, electrical, etc.), injection molded parts, micro-machined silicon/glass, and more.
Once biochemistry and microfluidics are optimized and the microfluidic cartridge functions identified, we arrive at the second critical inflection point: cartridge transfer.
Microfluidic requirements and the critical interfaces between the instrument and the disposable cartridge must be nailed down. A design freeze at this critical point is not only recommended, but required to keep the project on track.
Just as typical instrument development includes off-the-shelf (OTS) components, such as power supplies and LED displays, so too, should microfluidics. Consider the difference between creating an entirely new product from its component level on up versus integrating OTS components. This analogy is critical on the microfluidics and instrument side, but microfluidics is the gating factor.
Imagine for a minute that your microfluidics developer has few proven solutions for your application or hasn’t combined these functions in the past. Imagine also that once the cartridge is developed, it can’t be tested. It’s now up to the instrument developer to make it work, a task that may be beyond his capabilities.
Instead of using the trial and error learning curve, use existing technology whenever possible. Experienced microfluidic developers should have proven technology solutions and methods for testing and validating them. This should occur before passing the cartridge to the instrument developers, otherwise you might find yourself in a finger-pointing contest.
If instrument developers already understand the microfluidic requirements (third image in the above photo slider) and are proficient at incorporating these controls, then critical points in the transfer process are less likely to create problems. Instead of developing ways to solve cartridge inconsistencies, instrument developers can focus on the task at hand.
This leads to a third critical inflection point: system integration.
From an engineering and design-for-manufacturing perspective, the fewer mechanical parts there are and the lower the interconnection tolerances, the better. It is at this point the three development efforts converge to work together. Chemistries may need to be tweaked or modified to run correctly on the cartridge. Cartridge alignment may need to be keyed in the instrument instead of depending on an instrument edge for proper seating. Power requirements for handheld instruments may require better power management and less power consumption.
With thermocycling, the science, engineering, and system design must be coordinated to ensure a design feature in one component doesn’t add complexity or risk to another. Even the nature of the nucleic acid detection test can affect the system design and choice of materials, so it’s prudent to map out reasonable specifications on assay performance at the outset; choose the cartridge design, and interface to the detection and other electro-mechanical components early.
For example, the cartridge material must not react with the chemistry, so inert materials like polypropylene or polycarbonate might be used. The designers must understand how to mold it, attach various layers, best methods for making thermal contact, fluid containment during the thermocycling process, and providing optial clarity for detection.
System integration should be done in stages as sub-systems become available or can be simulated in software. Components for each sub-system should be tested and verified with its control electronics, then integrated into the instrument.
Software should provide a means for communication, early testing, and simulation even if hardware is not available. Biochemistry, microfluidics, and instrument teams should all be able to test their assumptions early and within the actual development process rather than toward the end.
Remember that exact protocols from the lab should not be expected to carry over to cartridge and instrument design. The number of cartridge functions and the precision in features in the cartridge should be minimized in order to keep cost and complexity down and performance up. Revenue models driven by cartridge sales can make or break a startup company if the disposable doesn’t provide sufficient margin.
Microfluidic cartridges need to be tested and verified before being transferred to the instrument developer, preferably with some knowledge of the fluid control components required and an understanding of the actuation routine. Otherwise, the project can be sidetracked with component specifications for the cartridge actuation routine instead of being focused on system integration issues. This can cause significant delays and frustration with trial-and-error processes attempting to solve multiple problems at once. With the right team, there will be a clear path to manufacturing scalability, and cost targets.
Off-the-shelf components in the instrument and proven microfluidic sub-systems should drive the development effort, using as much existing technology as possible. Custom-built modifications to either the cartridge or the instrument should be kept to a minimum.
POC diagnostic development is a complicated effort between multiple teams. The development is complete only when the system works reliably and cost effectively. Biochemisty, microfluidics, and instrument design must collaborate throughout the project to create a commercially successful POC diagnostic system. This is a difficult undertaking to accomplish internally, but very achievable through appropriate outsourcing.
Steve Maylish has been a part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences. After receiving his MBA in 2002, Maylish focused his business development skills on two boutique medical design/manufacturing firms that were acquired. Afterwards, he founded Maytrix Medical to provide business development to third-party engineering firms. Maylish has an A.S. degree in electronics from RETS Electronics Institute, a B.S. in business from the University of La Verne, and an M.B.A. from the University of California, Irvine.
Leanna M. Levine is an entrepreneur, technologist, and inventor. She founded ALine in 2003. Prior to founding ALine, Levine was engaged in new technology development in membrane separations, bioanalysis, and high throughput screening while a member of Monsanto’s corporate R&D. In 1998, she joined Spectrum Laboratories as director of manufacturing and product development. Levine earned her Ph.D. at Washington University, St. Louis (1986), and her bachelor of science degree in biochemistry and bachelor of arts in German from the University of Missouri, Columbia. In 2000, she chaired the Gordon Conference on Bioanalytical Sensors. She has co-authored a dozen publications and also several patents.