Mark Crawford, Contributing Writer10.16.13
Life’s pretty busy for tubing manufacturers these days. OEMs keep pushing the limits of manufacturing science with smaller diameters, thinner walls and more complex lumens to meet today’s clinical requirements, including less-invasive surgery and better patient outcomes. For example, tubing can get as small as 0.001 inches thick with an outside diameter of 0.010 inches. Combination devices that release pharmaceutical ingredients or are made from antimicrobial materials are also in high demand.
This constant quest for higher functionality is driving manufacturers away from the slower hand assembly of certain products (guide catheters, for example) toward methods that enable continuous extrusion of plastic with distinct physical properties (inside versus the outside layers, lumens with different qualities and along the length of the tube)—which saves on labor costs and increases quality.
Also, with increased regulatory oversight from various government agencies, OEMs want processes that are fully validated and meet regulatory expectations in full, which are constantly evolving. For example, the U.S. Food and Drug Administration (FDA), U.S. Securities and Exchange Commission, and the United Kingdom’s National Measurement Office are strengthening regulations pertaining to the composition and source of raw materials used in medical device tubing, as well as the controls on processing conditions that affect product cleanliness and contamination. The FDA also regulates compliance with guidance documents relating to the purification of water used in the extrusion process.
“We have seen the industry respond by taking steps with tubing manufacturers to ensure compliance with these new requirements, including certifications concerning the mineral content and material components used for colorants and pigments in medical device tubing,” said Robert LaDuca, CEO for Duke Empirical, a Santa Cruz, Calif.-based contract manufacturer specializing in finished catheter assemblies, catheter tubing components and custom medical extrusion.
Therefore, as regulations tighten and devices get smaller and more complex, tubing suppliers must stay ahead of the game and have the capability to deliver tubing in smaller dimensions, with very tight tolerances and highly validated processes. They also must be prepared to make the necessary capital investments in extrusion technology to stay competitive and meet or exceed OEM demands.
Increased Functionality
Customers are looking for increased functionality in interventional catheters systems for improved diagnostics and/or therapeutic delivery—at the same time they are pushing for reduced costs by consolidating components and eliminating assembly steps. Even though the catheter market is well developed and highly competitive, the rate of innovation in catheter devices continues to grow at a rapid pace.
“Since 2008, there has been a 170 percent increase in number of U.S. patents issued with ‘catheter’ in the title,” said Jim Dandeneau, CEO for Putnam Plastics Corporation, a Dayville, Conn.-based extruder of medical tubing. “Many of these are product improvements in traditional devices, such as angioplasty and guiding and imaging catheters. Patents associated with manufacturing or processing technologies are also significant.”
One way to increase functionality while reducing assembly steps is through the use of bump/taper tubing. This simplifies tubing designs and eliminates the need to connect tubes of different diameters.
“For example, more bump-tubing is being used to eliminate splicing or sleeving,” said Scott Nicora, vice president for NDH Medical Inc., a St. Petersburg, Fla.-based custom extruder for the medical device and life-science industries. “Paratubing is also in demand for eliminating sloppy leads or secondary gluing. These approaches can reduce costs 5 percent or more by eliminating secondary steps.”
Tubing also is being used as a conduit for data signal, electronic impulse, positioning device and other electronic transmission functions. The standard approach has been to add wires or electronic components in a post-assembly step. With cost reduction a constant state of mind, OEMs are asking tubing suppliers to co-extrude wires within the walls of the tubing to reduce assembly costs.
New England Catheter Corporation (NECC), a provider of custom medical extrusions in Lisbon, N.H., is getting proposals from companies that want to transmit electrical power to an electrosurgical device located at one end of the tube and/or they are trying to transmit/receive electrical signals to/from a digital imaging device or biomedical sensor. So, companies must incorporate power leads, micro-miniature coaxial cables and signal wires within the tube.
“We are able to run them longitudinally or spiral-wrapped within the wall of the tubing,” said Michael Boivin, manufacturing manager for NECC. “This aids in keeping the overall diameter of the tubing smaller and also maintains lumen patency throughout the length of the tube.”
Raumedic Inc., a Leesburg, Va.-based extruder of polymer and silicone components for the medical industry, provides similar
services with its own in-house technology.

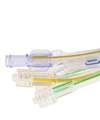
Miniaturization leads to complex devices with more lumens, variable shapes and smaller diameters. Photo courtesy of Raumedic Inc.
“From the cost standpoint, co-extrusion of wires will reduce labor/fabrication cost because a separate step is not required to feed the wires into empty lumens,” said Tom Moore, technical sales manager for Raumedic. “However, one has to be aware of the overall yield loss—scrapping tubing with embedded wire is much more expensive than scrapping tubing only. It really is a case-by-case basis when it comes to cost reduction.”
Of course, the drive for miniaturization is here to stay with smaller devices that are more functional, complex and contain more lumens of variable shapes with smaller diameters. These needs continue to push the limits of extrusion technology—tubing manufacturers must become adept with new equipment, a greater variety of engineered plastics with different characteristics and sophisticated quality-control systems that are constantly monitoring processing parameters, measuring dimensions and looking for defects. With miniaturized, minimally invasive devices that reach deeper into the body in more confined spaces, next-generation catheters are smaller in diameter yet provide the same functionality within the working channels of the device by maintaining lumen (or inside) dimensions. This means reducing wall thickness as much as possible.
“We’re in production now on a small five-lumen tube with absolutely critical dimensional requirements,” said Drew Rogers, vice president of sales and marketing for Specialty Silicone Fabricators (SSF), a Paso Robles, Calif.-based silicone extruder.
“Approximately 40 measurements are taken on each cross section and all must be within specification—it is highly challenging work.”
Raumedic has invested in new micro-extrusion technologies to meet demands for increasingly small dimensions and tight tolerances. “We now have the ability to extrude tubing with an inner diameter as small as 0.1 millimeter, wall thickness as small as 0.05 millimeter and tolerance as tight as plus or minus 0.02 millimeters,” said Moore. “We also have the capability to extrude up to four materials at once within our micro-extrusion equipment.”
An Array of Applications
Device manufacturers are asking for additional process controls to improve product quality and reduce the cost of manufacturing. They are especially looking for innovative solutions that meet their post-extrusion processing needs.
“Some of these applications include bonding balloons to PTCA (percutaneous transluminal coronary angioplasty) catheters, thermal processes for reflowing materials for making multi-durometer, steerable catheter delivery systems, and forming precise device curvatures to ease deployment of devices during cardiac and endoscopic applications,” said David Joyce, sales engineering manager for Vante, a Tucson, Ariz.-based supplier of medical device manufacturing equipment.
More miniaturized devices and tools are being used for minimally invasive surgical procedures. These can reduce the number of incisions and the size of the incisions for an increasing number of procedures. As a result, tubing manufacturers are being pressured to reduce outside tubing diameters, while still maintaining or increasing the functionality of the tubing.
“The goal is to make the inner diameter as large as possible while reducing the tubing wall and adding multi-lumen functionality,” said Boivin. “We are constantly trying to reduce tubing wall sizes. One of the challenges with thinner walls is that the tubing wants to kink when bent. The outside diameter will become rough and/or if there is any reinforcement within the wall, the braid or spiral might protrude through as well.”
Achieving these goals is challenging and requires a higher level of innovation. NECC has developed proprietary programmable logic controllers for its extruders and continuously monitors the inline extrusion process. Ultrasonic and laser precision-measurement equipment is used to monitor wall thickness, concentricity and diameter of tubing. Depending on the application, OEM specifications for reinforced thin walls also can be achieved with fine-wire (flat and round) spiral solutions.
Thousands of resin compounds have been engineered to provide specific physical properties such as enhanced strength, flexibility, pushability and lubricity. This wide range of material characteristics expands the design options for tubing and how it can be used in new devices. For example, even though polyvinyl chloride (PVC) has a well-established history in the medical device industry due to its versatility and cost, an increasingly recognized downside is its phthalate content and the health risk it presents. As a result, more companies are shifting toward non-phthalate PVC alternatives.
“Customers are inquiring about PVC replacements,” said Moore. “PVC-free alternatives function just as well as PVC, but the cost remains the major hurdle. Non-PVC materials are typically more expensive than PVC, making companies reluctant to change over. In addition to the lack of plasticizers that migrate from the material, many PVC alternatives use no chlorine chemistry in the synthesis of the polymers and also show excellent drug compatibility.”
Advanced Processes
Catheters are costly and challenging to manufacture due to complex shaft requirements, including a lubricious inner layer, braided stainless steel middle layer and variable-durometer outer layer. Product performance can vary due to poor bonding between the layers, or at the union of the outer layer segments, where hinge points occur.
The conventional approach is to select a substrate, place the braid over it, add sections of different durometer polymers and then encapsulate and reflow with heat shrink tubing—a labor-intensive process. Putnam Plastics has developed an extrusion technology called Tri-Tie that eliminates this traditional manual assembly of multiple components by creating a three-layer composite shaft, with maximum adhesion between the layers for improved performance.
“What is unique is our ability to place braids in a multi-lumen structure, with some lumens lined, and then coat that structure with our Tri-Tie variable flexibility continuous process,” said Dandeneau. “It also provides a more gradual transition of the varying durometer outer layer, without bonding or hinge points.”
NECC engineers use fluid dynamic analysis software to evaluate the flow of resins in the extrusion tooling, prior to the tooling being built. This saves time and money by minimizing the number of tooling iterations. The result is properly designed extrusion tooling in a shorter amount of time. Fluid dynamic analysis is used to minimize the development time necessary for producing parallel tubing, where two or more tubes are extruded parallel to each other.
“This type of extrusion requires tooling that is specifically designed for each product,” said Boivin. “The process often requires a number of tooling iterations to get the correct resin flow to provide the necessary extrusion profile. It also requires both engineering and machine shop resources. Investment in fluid dynamic analysis software has reduced the tooling iterations. What might have taken three tooling iterations in the past can often be accomplished in the first prototype run with fluid dynamic analysis.”
Specialty Silicone Fabricators has developed a system by which the die and mandrel (the die forms the outer surface of the tube while the mandrel creates the inner shape) can be moved or exchanged “on the fly” during the extrusion process. Called GeoTrans, the process improves component manufacturability and reduces costs, according to the company.
For example, many medical products, including cardiac stimulation devices, use silicone tubing as an electrical insulator. Typically these devices merge two discrete conducting wires at a connector, and then redirect these conductors through a single-lumen tube.
“Historically this involved precision fixturing of the various tubes, followed by over-molding of the central connector,” said Rogers. “With GeoTrans, the inner diameter/outer diameter tube, flat-transition hub and two single-lumen tubes are manufactured in a single and continuous process, eliminating costly secondary assembly time and costs.”
Being Part of the Team
As the complexity and technical performance requirements for tubing increases, it is increasingly important for the extrusion producer to work closely with the OEM to optimize a solution that meets all the technical requirements, including manufacturing stability within processing and material limits. It is the skill and expertise of the processor in choosing the parameters that will produce the best tubing with the desired output properties. Without early and frequent discussions between the tubing supplier and the device development and manufacturing teams to fully analyze the OEM’s goals for product performance, opportunities for optimization of material properties are often missed. Early collaboration leads to new innovative approaches, technological improvements and best practices.
“For example,” said LaDuca, “consider a customer’s requirement for a thin-wall extrusion that will be used as a pusher in a delivery device. During device deployment, the pusher tubing is under a compressive load and should not buckle or shorten. Having this knowledge in advance, the processor can select parameters such as the area draw-down ratio or the distance from the extruder crosshead tooling to the water bath.”
Varying these two parameters creates geometrically identical tubing, but with much different functional performances.
“Increasing linear stress by inducing a higher degree of linear molecular orientation through a higher draw-down ratio results in tubing with higher tensile strength, greater stiffness and column strength, but comes at a cost of reduced bend radius or kink resistance,” he said. “If the customer requires more flexibility or radial strength for traversing tortuous pathways without kinking, or to hold pressure, then the extrusion process would need to be set up with a different set of parameters to achieve higher hoop strength and elongation within the same geometrical profile.”
Some recent successes that evolved from early discussions with OEM design teams include:
The trend toward more integrated, less-invasive medical devices to lower patient risk and shorten the recovery time following surgery will continue. OEMs are eager to bring smaller, more complex and less-invasive devices to the marketplace with shorter developmental cycles and lower costs. This of course translates into the need for smaller parts, extremely thin-walled tubing and tighter tolerances. A few years ago dimensional tolerances of plus or minus 0.001 inch were the standard. Today, however, design engineers are now requesting tolerances of 0.0005 inch and even less.
Medical device manufacturers and their suppliers will have to stay on the leading edge of technology, material science and best practices to meet these evolving manufacturing challenges. This includes using design of experiments, production data and analysis software to predict and identify the critical processing parameters needed to produce these complex tubing solutions.
OEMs are intensely focused on functionality, speed to market, and cost. As a result, tubing continues to push the envelope as more advanced materials and innovative technologies are developed. This, of course, drives tubing suppliers to increase their competitiveness by doing whatever they can to add value and differentiate themselves from the crowd.
“For example, tubing suppliers are challenging each other with reduced ‘trial’ costs and quick turnaround times for prototype samples,” said Moore, adding that supplying the OEM with a functioning prototype is only part of the game.
“The tubing supplier must be able to turn this prototype into a working product that is very high quality, produced with a validated process and cost-effective,” he said. “Having the ability to develop innovative products, the flexibility to offer short turnaround times for samples and strong quality principles are key factors for tubing success within the medical industry.”
Mark Crawford is a full-time freelance business, marketing and communications writer based in Madison, Wis. He can be reached at mark.crawford@charter.net.
This constant quest for higher functionality is driving manufacturers away from the slower hand assembly of certain products (guide catheters, for example) toward methods that enable continuous extrusion of plastic with distinct physical properties (inside versus the outside layers, lumens with different qualities and along the length of the tube)—which saves on labor costs and increases quality.
Also, with increased regulatory oversight from various government agencies, OEMs want processes that are fully validated and meet regulatory expectations in full, which are constantly evolving. For example, the U.S. Food and Drug Administration (FDA), U.S. Securities and Exchange Commission, and the United Kingdom’s National Measurement Office are strengthening regulations pertaining to the composition and source of raw materials used in medical device tubing, as well as the controls on processing conditions that affect product cleanliness and contamination. The FDA also regulates compliance with guidance documents relating to the purification of water used in the extrusion process.
“We have seen the industry respond by taking steps with tubing manufacturers to ensure compliance with these new requirements, including certifications concerning the mineral content and material components used for colorants and pigments in medical device tubing,” said Robert LaDuca, CEO for Duke Empirical, a Santa Cruz, Calif.-based contract manufacturer specializing in finished catheter assemblies, catheter tubing components and custom medical extrusion.
Therefore, as regulations tighten and devices get smaller and more complex, tubing suppliers must stay ahead of the game and have the capability to deliver tubing in smaller dimensions, with very tight tolerances and highly validated processes. They also must be prepared to make the necessary capital investments in extrusion technology to stay competitive and meet or exceed OEM demands.
Increased Functionality
Customers are looking for increased functionality in interventional catheters systems for improved diagnostics and/or therapeutic delivery—at the same time they are pushing for reduced costs by consolidating components and eliminating assembly steps. Even though the catheter market is well developed and highly competitive, the rate of innovation in catheter devices continues to grow at a rapid pace.
“Since 2008, there has been a 170 percent increase in number of U.S. patents issued with ‘catheter’ in the title,” said Jim Dandeneau, CEO for Putnam Plastics Corporation, a Dayville, Conn.-based extruder of medical tubing. “Many of these are product improvements in traditional devices, such as angioplasty and guiding and imaging catheters. Patents associated with manufacturing or processing technologies are also significant.”
One way to increase functionality while reducing assembly steps is through the use of bump/taper tubing. This simplifies tubing designs and eliminates the need to connect tubes of different diameters.
“For example, more bump-tubing is being used to eliminate splicing or sleeving,” said Scott Nicora, vice president for NDH Medical Inc., a St. Petersburg, Fla.-based custom extruder for the medical device and life-science industries. “Paratubing is also in demand for eliminating sloppy leads or secondary gluing. These approaches can reduce costs 5 percent or more by eliminating secondary steps.”
Tubing also is being used as a conduit for data signal, electronic impulse, positioning device and other electronic transmission functions. The standard approach has been to add wires or electronic components in a post-assembly step. With cost reduction a constant state of mind, OEMs are asking tubing suppliers to co-extrude wires within the walls of the tubing to reduce assembly costs.
New England Catheter Corporation (NECC), a provider of custom medical extrusions in Lisbon, N.H., is getting proposals from companies that want to transmit electrical power to an electrosurgical device located at one end of the tube and/or they are trying to transmit/receive electrical signals to/from a digital imaging device or biomedical sensor. So, companies must incorporate power leads, micro-miniature coaxial cables and signal wires within the tube.
“We are able to run them longitudinally or spiral-wrapped within the wall of the tubing,” said Michael Boivin, manufacturing manager for NECC. “This aids in keeping the overall diameter of the tubing smaller and also maintains lumen patency throughout the length of the tube.”
Raumedic Inc., a Leesburg, Va.-based extruder of polymer and silicone components for the medical industry, provides similar
services with its own in-house technology.
Miniaturization leads to complex devices with more lumens, variable shapes and smaller diameters. Photo courtesy of Raumedic Inc.
Of course, the drive for miniaturization is here to stay with smaller devices that are more functional, complex and contain more lumens of variable shapes with smaller diameters. These needs continue to push the limits of extrusion technology—tubing manufacturers must become adept with new equipment, a greater variety of engineered plastics with different characteristics and sophisticated quality-control systems that are constantly monitoring processing parameters, measuring dimensions and looking for defects. With miniaturized, minimally invasive devices that reach deeper into the body in more confined spaces, next-generation catheters are smaller in diameter yet provide the same functionality within the working channels of the device by maintaining lumen (or inside) dimensions. This means reducing wall thickness as much as possible.
“We’re in production now on a small five-lumen tube with absolutely critical dimensional requirements,” said Drew Rogers, vice president of sales and marketing for Specialty Silicone Fabricators (SSF), a Paso Robles, Calif.-based silicone extruder.
“Approximately 40 measurements are taken on each cross section and all must be within specification—it is highly challenging work.”
Raumedic has invested in new micro-extrusion technologies to meet demands for increasingly small dimensions and tight tolerances. “We now have the ability to extrude tubing with an inner diameter as small as 0.1 millimeter, wall thickness as small as 0.05 millimeter and tolerance as tight as plus or minus 0.02 millimeters,” said Moore. “We also have the capability to extrude up to four materials at once within our micro-extrusion equipment.”
An Array of Applications
Device manufacturers are asking for additional process controls to improve product quality and reduce the cost of manufacturing. They are especially looking for innovative solutions that meet their post-extrusion processing needs.
“Some of these applications include bonding balloons to PTCA (percutaneous transluminal coronary angioplasty) catheters, thermal processes for reflowing materials for making multi-durometer, steerable catheter delivery systems, and forming precise device curvatures to ease deployment of devices during cardiac and endoscopic applications,” said David Joyce, sales engineering manager for Vante, a Tucson, Ariz.-based supplier of medical device manufacturing equipment.
More miniaturized devices and tools are being used for minimally invasive surgical procedures. These can reduce the number of incisions and the size of the incisions for an increasing number of procedures. As a result, tubing manufacturers are being pressured to reduce outside tubing diameters, while still maintaining or increasing the functionality of the tubing.
“The goal is to make the inner diameter as large as possible while reducing the tubing wall and adding multi-lumen functionality,” said Boivin. “We are constantly trying to reduce tubing wall sizes. One of the challenges with thinner walls is that the tubing wants to kink when bent. The outside diameter will become rough and/or if there is any reinforcement within the wall, the braid or spiral might protrude through as well.”
Achieving these goals is challenging and requires a higher level of innovation. NECC has developed proprietary programmable logic controllers for its extruders and continuously monitors the inline extrusion process. Ultrasonic and laser precision-measurement equipment is used to monitor wall thickness, concentricity and diameter of tubing. Depending on the application, OEM specifications for reinforced thin walls also can be achieved with fine-wire (flat and round) spiral solutions.
Thousands of resin compounds have been engineered to provide specific physical properties such as enhanced strength, flexibility, pushability and lubricity. This wide range of material characteristics expands the design options for tubing and how it can be used in new devices. For example, even though polyvinyl chloride (PVC) has a well-established history in the medical device industry due to its versatility and cost, an increasingly recognized downside is its phthalate content and the health risk it presents. As a result, more companies are shifting toward non-phthalate PVC alternatives.
“Customers are inquiring about PVC replacements,” said Moore. “PVC-free alternatives function just as well as PVC, but the cost remains the major hurdle. Non-PVC materials are typically more expensive than PVC, making companies reluctant to change over. In addition to the lack of plasticizers that migrate from the material, many PVC alternatives use no chlorine chemistry in the synthesis of the polymers and also show excellent drug compatibility.”
Advanced Processes
Catheters are costly and challenging to manufacture due to complex shaft requirements, including a lubricious inner layer, braided stainless steel middle layer and variable-durometer outer layer. Product performance can vary due to poor bonding between the layers, or at the union of the outer layer segments, where hinge points occur.
The conventional approach is to select a substrate, place the braid over it, add sections of different durometer polymers and then encapsulate and reflow with heat shrink tubing—a labor-intensive process. Putnam Plastics has developed an extrusion technology called Tri-Tie that eliminates this traditional manual assembly of multiple components by creating a three-layer composite shaft, with maximum adhesion between the layers for improved performance.
“What is unique is our ability to place braids in a multi-lumen structure, with some lumens lined, and then coat that structure with our Tri-Tie variable flexibility continuous process,” said Dandeneau. “It also provides a more gradual transition of the varying durometer outer layer, without bonding or hinge points.”
NECC engineers use fluid dynamic analysis software to evaluate the flow of resins in the extrusion tooling, prior to the tooling being built. This saves time and money by minimizing the number of tooling iterations. The result is properly designed extrusion tooling in a shorter amount of time. Fluid dynamic analysis is used to minimize the development time necessary for producing parallel tubing, where two or more tubes are extruded parallel to each other.
“This type of extrusion requires tooling that is specifically designed for each product,” said Boivin. “The process often requires a number of tooling iterations to get the correct resin flow to provide the necessary extrusion profile. It also requires both engineering and machine shop resources. Investment in fluid dynamic analysis software has reduced the tooling iterations. What might have taken three tooling iterations in the past can often be accomplished in the first prototype run with fluid dynamic analysis.”
Specialty Silicone Fabricators has developed a system by which the die and mandrel (the die forms the outer surface of the tube while the mandrel creates the inner shape) can be moved or exchanged “on the fly” during the extrusion process. Called GeoTrans, the process improves component manufacturability and reduces costs, according to the company.
For example, many medical products, including cardiac stimulation devices, use silicone tubing as an electrical insulator. Typically these devices merge two discrete conducting wires at a connector, and then redirect these conductors through a single-lumen tube.
“Historically this involved precision fixturing of the various tubes, followed by over-molding of the central connector,” said Rogers. “With GeoTrans, the inner diameter/outer diameter tube, flat-transition hub and two single-lumen tubes are manufactured in a single and continuous process, eliminating costly secondary assembly time and costs.”
Being Part of the Team
As the complexity and technical performance requirements for tubing increases, it is increasingly important for the extrusion producer to work closely with the OEM to optimize a solution that meets all the technical requirements, including manufacturing stability within processing and material limits. It is the skill and expertise of the processor in choosing the parameters that will produce the best tubing with the desired output properties. Without early and frequent discussions between the tubing supplier and the device development and manufacturing teams to fully analyze the OEM’s goals for product performance, opportunities for optimization of material properties are often missed. Early collaboration leads to new innovative approaches, technological improvements and best practices.
“For example,” said LaDuca, “consider a customer’s requirement for a thin-wall extrusion that will be used as a pusher in a delivery device. During device deployment, the pusher tubing is under a compressive load and should not buckle or shorten. Having this knowledge in advance, the processor can select parameters such as the area draw-down ratio or the distance from the extruder crosshead tooling to the water bath.”
Varying these two parameters creates geometrically identical tubing, but with much different functional performances.
“Increasing linear stress by inducing a higher degree of linear molecular orientation through a higher draw-down ratio results in tubing with higher tensile strength, greater stiffness and column strength, but comes at a cost of reduced bend radius or kink resistance,” he said. “If the customer requires more flexibility or radial strength for traversing tortuous pathways without kinking, or to hold pressure, then the extrusion process would need to be set up with a different set of parameters to achieve higher hoop strength and elongation within the same geometrical profile.”
Some recent successes that evolved from early discussions with OEM design teams include:
- An NDH Medical customer asked the company to help improve the customer’s process of hand-assembling thin-walled tubing over a metal coil. NDH engineers designed a system that automatically jacketed the coil, removing the assembly step,
- reducing labor required and reducing part count.
- Vante recently concluded a project where it recommended altering the product design on a polytetrafluoroethylene (PTFE)-lined steerable introducer. By making slight adjustments in the PTFE liner diameter and axial position, manufacturing yields were increased from about 50 percent to almost 99 percent,
- significantly reducing cost.
- Raumedic has developed material formulations containing antimicrobial additives that can be tailored to individual customer requirements and are compatible with a wide variety of base materials. According to company officials, mixing these special additives into the melt before extrusion begins eliminates the need to coat the extruded tubing with an antimicrobial solution.
- NECC manufactures a variety of braid-reinforced multi-lumen shafts, including a multi-durometer shaft. The process can be adjusted to meet a wide range of complex tubing requirements. The braid can be customized to provide specific torque transmissions along the length of the shaft; the multi-durometer allows for varying degrees of flexibility, according to the company.
The trend toward more integrated, less-invasive medical devices to lower patient risk and shorten the recovery time following surgery will continue. OEMs are eager to bring smaller, more complex and less-invasive devices to the marketplace with shorter developmental cycles and lower costs. This of course translates into the need for smaller parts, extremely thin-walled tubing and tighter tolerances. A few years ago dimensional tolerances of plus or minus 0.001 inch were the standard. Today, however, design engineers are now requesting tolerances of 0.0005 inch and even less.
Medical device manufacturers and their suppliers will have to stay on the leading edge of technology, material science and best practices to meet these evolving manufacturing challenges. This includes using design of experiments, production data and analysis software to predict and identify the critical processing parameters needed to produce these complex tubing solutions.
OEMs are intensely focused on functionality, speed to market, and cost. As a result, tubing continues to push the envelope as more advanced materials and innovative technologies are developed. This, of course, drives tubing suppliers to increase their competitiveness by doing whatever they can to add value and differentiate themselves from the crowd.
“For example, tubing suppliers are challenging each other with reduced ‘trial’ costs and quick turnaround times for prototype samples,” said Moore, adding that supplying the OEM with a functioning prototype is only part of the game.
“The tubing supplier must be able to turn this prototype into a working product that is very high quality, produced with a validated process and cost-effective,” he said. “Having the ability to develop innovative products, the flexibility to offer short turnaround times for samples and strong quality principles are key factors for tubing success within the medical industry.”
Mark Crawford is a full-time freelance business, marketing and communications writer based in Madison, Wis. He can be reached at mark.crawford@charter.net.