07.26.18
$5.8 Billion ($31.7B total)
KEY EXECUTIVES
Inge. G. Thulin, Chairman of the Board, President, and CEO
Hak Cheol Shin, Vice Chair and Exec. VP
Michael F. Roman, COO and Exec. VP
Nicholas C. Gangestad, Sr. VP and CFO
Michael G. Vale, Exec. VP, Health Care Business Group
John P. Banovetz, Sr. VP, R&D and CTO
Julie L. Bushman, Exec. VP, International Operations
Ivan K. Fong, Sr. VP, Legal Affairs and General Counsel
Paul A. Keel, Sr. VP, Business Development and Marketing-Sales
Jon T. Lindekugel, Sr. VP, Supply Chain
Kristen M. Ludgate, Sr. VP, Corporate Communications and Enterprise Services
Marlene M. McGrath, Sr. VP, Human Resources
NO. OF EMPLOYEES: 91,536
GLOBAL HEADQUARTERS: St. Paul, Minn.
Ever since CMS announced it would not reimburse hospitals or medical facilities for healthcare-associated infections, the attention being paid to their prevention has become significant. The decision has resulted in the launch of infection-control technologies such as UV sterilization robots, and an increased reliance on single-use products. Surgical instrumentation and other reusable devices, however, still require stringent sterilization processes that must eliminate any potential threat of infection.
In 2017, 3M responded to this need with the release of its new biological indicator test for vaporized hydrogen peroxide sterilization (VH2O2). The 24-minute test (approximately 10 times faster than the company’s previous four-hour test, released in 2016) offers sterility assurance to healthcare facilities attempting to eliminate containments from surgical instruments.
“We’ve seen overwhelming market excitement about this system since we announced 510(k) submission in May,” Ericka Lutz, 3M global marketing manager for sterilization, said in a company announcement noting the release of the 3M Attest Rapid Readout Biological Indicator (BI) System for VH2O2. “The common reaction we’re hearing is, ‘If our hospital is able to improve efficiencies and enhance patient safety, it’s a clear win-win decision.’”
Further, healthcare provider customers already utilizing the four-hour version of the test were granted free software upgrades to the newer system, according to an article about the technology in the Star Tribune. Lutz told the paper, “We want all customers to be able to benefit from our innovation and use the speed of our system to help increase patient safety as fast as possible.”
That message mirrors the thoughts of 3M’s Inge G. Thulin, chairman of the board, president, and CEO, in his letter to shareholders at the start of the company’s 2017 annual report. “In partnership with our customers, we use science to improve lives and help solve society’s toughest challenges—from improving air quality and worker safety, to advancing healthcare and enabling the transportation of tomorrow.”
ANALYST INSIGHTS: 3M remains a steady player in healthcare in 2018. Watch for continued, consistent organic and small, inorganic growth with no major drama. They have excellent leadership in Health Care and will continue to perform well for the short and long term.
That vision has led to the company enjoying very consistent performance over a number of years. The corporate entity saw sales near $32 billion for the second time in four years ($31.7 billion in 2017, almost exceeding 2014’s $31.8 billion), which represented a 5.1 percent bump from 2016. The company also ensured continued future success with an investment of over $5 billion toward research and development across all divisions, capital expenditure, and acquisitions. Maintaining a clear focus on that future success is also apparent in 3M’s ongoing strategy of focusing on strategic interests and streamlining operations. Since 2012, the firm has realigned from six business groups to five and from 40 businesses to 24. One such move occurred last December, when the firm sent its Communications markets division to Corning Inc. for a price of $900 million. The segment provided connectivity and cabling solutions for the telecommunications industry.
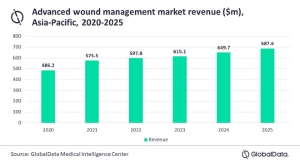
The Health Care group is the company’s third largest segment at $5.8 billion in 2017 sales [behind Industrial’s $10.9 billion and Safety and Graphics’ $6.1 billion; Electronics and Energy ($5.2 billion) and Consumer ($4.6 billion) represent the fourth and fifth segments respectively]. The segment saw a modest 4.4 percent increase over 2016, which was a result of sales across all regions, with Asia Pacific (8.6 percent) and Latin America/Canada (9.3 percent) posting the largest gains. The group’s $5.8 billion in sales represents an 18.4 percent contribution to the company’s total figure.
3M’s Health Care segment customers originate from a wide variety of medical-related markets, from medical clinics and hospitals to dental/orthodontic practitioners to health information systems, as well as others. The company’s products indicated for these sectors include medical and surgical supplies, skin health and infection prevention products, oral care solutions (dental and orthodontic products), health information systems, inhalation and transdermal drug delivery systems, and food safety products, which saw an influx of new offerings from a targeted acquisition.
The purchase of Elution Technologies by 3M was announced on Sept. 7, 2017. The firm is a manufacturer of test kits that can be used to ensure products are free of potentially harmful allergens, such as peanuts, soy, and milk. Allergic reactions can pose a significant health risk for those susceptible to a particular allergen, which according to a 2011 Journal of Clinical Immunology study, resulted in a person in the United States being rushed to the emergency room every three minutes. In some instances, it was the result of exposure to a food product that was never intended to be present.
“Elution Technologies’ test kits offer proven technology with an easy-to-use design that delivers fast and accurate results for companies offering peanut-free, gluten-free, and other specialized foods for people with certain sensitivities and allergies,” said Polly Foss, general manager of 3M Food Safety. “We are pleased to add this technology to our broader food safety offering, and extend these important solutions to food processing companies across the globe.”
In sectors slightly more affiliated with traditional healthcare, 3M’s expertise in the adhesive space has led it to offer device makers options for keeping wearable medical technologies in place. Last August, it grew this line with the addition of the ISO:10993 and ISO:10993-10 compliant 4076 Extended Wear Medical Tape, which provides medical device manufacturers a long-term wear, acrylic-based adhesive solution designed to increase patient comfort.
“Sticking to skin presents a major challenge to the medical device industry,” said Diana Eitzman, Ph.D., director of agile commercialization, 3M Critical and Chronic Care Solutions Division. “By equipping our customers with the latest adhesive technology, we’re giving them the power to solve their toughest design challenges and positively impact patients’ lives globally.”
Leveraging the aforementioned focus on reducing infections with adhesive expertise, 3M experienced a serendipitous FDA 510(k) clearance of another product. The Tegaderm CHG I.V.
Securement Dressing gained the agency’s OK to expand its indications to reduce catheter-related bloodstream infection (CRBSI). Following on the heels of the June clearance, in November, the Centers for Disease Control (CDC) updated its recommendation regarding catheter-related infections. The Center called for the use of chlorhexidine-impregnated dressings for reducing CRBSI and catheter-associated bloodstream infections. At the time, the transparent dressing was the only one of its kind indicated for such use.
“The CDC’s evidence-based recommendations elevate current best practices in reducing life-threatening and costly bloodstream infections. The revisions highlight the strong clinical data that supports use of Tegaderm CHG I.V. Securement Dressing worldwide,” explained Pat Parks, M.D., Ph.D., medical director for 3M Critical and Chronic Care Solutions Division. “At 3M, our goal is zero bloodstream infections. We’ll keep innovating and educating to make that future possible.”
The dressing wasn’t the only technology 3M offered to clinicians and healthcare providers in the fight against catheter-related infections. Earlier in 2017, the company introduced its Curos Stopper Disinfecting Cap for Open Female Luers to help clinicians ensure all intraluminal vascular access points can be protected through passive disinfection. The caps can disinfect in one minute and provide protection for up to 7 days (if not removed). Further, the cap’s bright red color helps clinicians verify a port is clean at a glance and make disinfection compliance easy to measure.
Outside of the product announcements for 3M, the company’s 2017 fiscal year was unfortunately bookended by two more somber events. First, in January, the company noted that former chairman and CEO Livio D. “Desi” DeSimone—who led the company from 1991 to 2001—had died. Having celebrated a 43-year career with 3M, DeSimone held executive positions with most of the firm’s business sectors and was also area vice president for Latin America.
“Desi was a bold leader who courageously guided 3M through the turbulent economic decade of the 1990s. During his tenure as chairman and CEO, he strengthened 3M’s portfolio through his actions including the spinoff of the imaging systems business. He made long-term investments in core technology platforms, such as microreplication, that today are used broadly across our enterprise. Desi was a champion of the environment and demonstrated an unwavering commitment to sustainable business practices for 3M and the community,” Thulin stated in a company release.
At the end of the year, in December, the company filed a patent infringement lawsuit against Kerr Corporation. The lawsuit alleged that Kerr’s SonicFill 2 and Harmonize dental composite materials infringe on patent rights directed to nanotechnology used in 3M’s Filtek Supreme universal dental restoratives. No conclusion has yet been announced regarding the legal entanglement.
KEY EXECUTIVES
Inge. G. Thulin, Chairman of the Board, President, and CEO
Hak Cheol Shin, Vice Chair and Exec. VP
Michael F. Roman, COO and Exec. VP
Nicholas C. Gangestad, Sr. VP and CFO
Michael G. Vale, Exec. VP, Health Care Business Group
John P. Banovetz, Sr. VP, R&D and CTO
Julie L. Bushman, Exec. VP, International Operations
Ivan K. Fong, Sr. VP, Legal Affairs and General Counsel
Paul A. Keel, Sr. VP, Business Development and Marketing-Sales
Jon T. Lindekugel, Sr. VP, Supply Chain
Kristen M. Ludgate, Sr. VP, Corporate Communications and Enterprise Services
Marlene M. McGrath, Sr. VP, Human Resources
NO. OF EMPLOYEES: 91,536
GLOBAL HEADQUARTERS: St. Paul, Minn.
Ever since CMS announced it would not reimburse hospitals or medical facilities for healthcare-associated infections, the attention being paid to their prevention has become significant. The decision has resulted in the launch of infection-control technologies such as UV sterilization robots, and an increased reliance on single-use products. Surgical instrumentation and other reusable devices, however, still require stringent sterilization processes that must eliminate any potential threat of infection.
In 2017, 3M responded to this need with the release of its new biological indicator test for vaporized hydrogen peroxide sterilization (VH2O2). The 24-minute test (approximately 10 times faster than the company’s previous four-hour test, released in 2016) offers sterility assurance to healthcare facilities attempting to eliminate containments from surgical instruments.
“We’ve seen overwhelming market excitement about this system since we announced 510(k) submission in May,” Ericka Lutz, 3M global marketing manager for sterilization, said in a company announcement noting the release of the 3M Attest Rapid Readout Biological Indicator (BI) System for VH2O2. “The common reaction we’re hearing is, ‘If our hospital is able to improve efficiencies and enhance patient safety, it’s a clear win-win decision.’”
Further, healthcare provider customers already utilizing the four-hour version of the test were granted free software upgrades to the newer system, according to an article about the technology in the Star Tribune. Lutz told the paper, “We want all customers to be able to benefit from our innovation and use the speed of our system to help increase patient safety as fast as possible.”
That message mirrors the thoughts of 3M’s Inge G. Thulin, chairman of the board, president, and CEO, in his letter to shareholders at the start of the company’s 2017 annual report. “In partnership with our customers, we use science to improve lives and help solve society’s toughest challenges—from improving air quality and worker safety, to advancing healthcare and enabling the transportation of tomorrow.”
ANALYST INSIGHTS: 3M remains a steady player in healthcare in 2018. Watch for continued, consistent organic and small, inorganic growth with no major drama. They have excellent leadership in Health Care and will continue to perform well for the short and long term.
—Dave Sheppard, Co-Founder and Principal, MedWorld Advisors
That vision has led to the company enjoying very consistent performance over a number of years. The corporate entity saw sales near $32 billion for the second time in four years ($31.7 billion in 2017, almost exceeding 2014’s $31.8 billion), which represented a 5.1 percent bump from 2016. The company also ensured continued future success with an investment of over $5 billion toward research and development across all divisions, capital expenditure, and acquisitions. Maintaining a clear focus on that future success is also apparent in 3M’s ongoing strategy of focusing on strategic interests and streamlining operations. Since 2012, the firm has realigned from six business groups to five and from 40 businesses to 24. One such move occurred last December, when the firm sent its Communications markets division to Corning Inc. for a price of $900 million. The segment provided connectivity and cabling solutions for the telecommunications industry.
The Health Care group is the company’s third largest segment at $5.8 billion in 2017 sales [behind Industrial’s $10.9 billion and Safety and Graphics’ $6.1 billion; Electronics and Energy ($5.2 billion) and Consumer ($4.6 billion) represent the fourth and fifth segments respectively]. The segment saw a modest 4.4 percent increase over 2016, which was a result of sales across all regions, with Asia Pacific (8.6 percent) and Latin America/Canada (9.3 percent) posting the largest gains. The group’s $5.8 billion in sales represents an 18.4 percent contribution to the company’s total figure.
3M’s Health Care segment customers originate from a wide variety of medical-related markets, from medical clinics and hospitals to dental/orthodontic practitioners to health information systems, as well as others. The company’s products indicated for these sectors include medical and surgical supplies, skin health and infection prevention products, oral care solutions (dental and orthodontic products), health information systems, inhalation and transdermal drug delivery systems, and food safety products, which saw an influx of new offerings from a targeted acquisition.
The purchase of Elution Technologies by 3M was announced on Sept. 7, 2017. The firm is a manufacturer of test kits that can be used to ensure products are free of potentially harmful allergens, such as peanuts, soy, and milk. Allergic reactions can pose a significant health risk for those susceptible to a particular allergen, which according to a 2011 Journal of Clinical Immunology study, resulted in a person in the United States being rushed to the emergency room every three minutes. In some instances, it was the result of exposure to a food product that was never intended to be present.
“Elution Technologies’ test kits offer proven technology with an easy-to-use design that delivers fast and accurate results for companies offering peanut-free, gluten-free, and other specialized foods for people with certain sensitivities and allergies,” said Polly Foss, general manager of 3M Food Safety. “We are pleased to add this technology to our broader food safety offering, and extend these important solutions to food processing companies across the globe.”
In sectors slightly more affiliated with traditional healthcare, 3M’s expertise in the adhesive space has led it to offer device makers options for keeping wearable medical technologies in place. Last August, it grew this line with the addition of the ISO:10993 and ISO:10993-10 compliant 4076 Extended Wear Medical Tape, which provides medical device manufacturers a long-term wear, acrylic-based adhesive solution designed to increase patient comfort.
“Sticking to skin presents a major challenge to the medical device industry,” said Diana Eitzman, Ph.D., director of agile commercialization, 3M Critical and Chronic Care Solutions Division. “By equipping our customers with the latest adhesive technology, we’re giving them the power to solve their toughest design challenges and positively impact patients’ lives globally.”
Leveraging the aforementioned focus on reducing infections with adhesive expertise, 3M experienced a serendipitous FDA 510(k) clearance of another product. The Tegaderm CHG I.V.
Securement Dressing gained the agency’s OK to expand its indications to reduce catheter-related bloodstream infection (CRBSI). Following on the heels of the June clearance, in November, the Centers for Disease Control (CDC) updated its recommendation regarding catheter-related infections. The Center called for the use of chlorhexidine-impregnated dressings for reducing CRBSI and catheter-associated bloodstream infections. At the time, the transparent dressing was the only one of its kind indicated for such use.
“The CDC’s evidence-based recommendations elevate current best practices in reducing life-threatening and costly bloodstream infections. The revisions highlight the strong clinical data that supports use of Tegaderm CHG I.V. Securement Dressing worldwide,” explained Pat Parks, M.D., Ph.D., medical director for 3M Critical and Chronic Care Solutions Division. “At 3M, our goal is zero bloodstream infections. We’ll keep innovating and educating to make that future possible.”
The dressing wasn’t the only technology 3M offered to clinicians and healthcare providers in the fight against catheter-related infections. Earlier in 2017, the company introduced its Curos Stopper Disinfecting Cap for Open Female Luers to help clinicians ensure all intraluminal vascular access points can be protected through passive disinfection. The caps can disinfect in one minute and provide protection for up to 7 days (if not removed). Further, the cap’s bright red color helps clinicians verify a port is clean at a glance and make disinfection compliance easy to measure.
Outside of the product announcements for 3M, the company’s 2017 fiscal year was unfortunately bookended by two more somber events. First, in January, the company noted that former chairman and CEO Livio D. “Desi” DeSimone—who led the company from 1991 to 2001—had died. Having celebrated a 43-year career with 3M, DeSimone held executive positions with most of the firm’s business sectors and was also area vice president for Latin America.
“Desi was a bold leader who courageously guided 3M through the turbulent economic decade of the 1990s. During his tenure as chairman and CEO, he strengthened 3M’s portfolio through his actions including the spinoff of the imaging systems business. He made long-term investments in core technology platforms, such as microreplication, that today are used broadly across our enterprise. Desi was a champion of the environment and demonstrated an unwavering commitment to sustainable business practices for 3M and the community,” Thulin stated in a company release.
At the end of the year, in December, the company filed a patent infringement lawsuit against Kerr Corporation. The lawsuit alleged that Kerr’s SonicFill 2 and Harmonize dental composite materials infringe on patent rights directed to nanotechnology used in 3M’s Filtek Supreme universal dental restoratives. No conclusion has yet been announced regarding the legal entanglement.