Michael Barbella, Managing Editor01.18.22
Timing truly is central to business success.
Microsoft, for example, could very well have been the world’s first trillion-dollar company had the public warmed to its tablet at the millennium’s dawning. Similarly, the world was not quite ready to embrace the future when Bell Labs unveiled its Picturephone in 1964 (imagine how different life would be today had it become commonplace back then).
Surprisingly, biotechnology firm Vaxxas hasn’t encountered that same dead end despite its invention being a bit ahead of its own time. The privately-held company is developing needle-free vaccination technology originally hatched by the Australian Institute of Bioengineering & Nanotechnology at The University of Queensland.
The technology uses a proprietary high-density micro-projection array patch (HD-MAP) to streamline and improve vaccine delivery. The postage stamp-sized silicon patch contains tens of thousands of projections 200-300 microns in length that release vaccine antigens directly to immune cells sitting just below the skin’s surface.
Preclinical studies have shown the Nanopatch to be considerably more effective than conventional vaccine delivery systems, with as little as 1/100th of its dose eliciting the same immune response as a “full” portion through intramuscular injection. Moreover, Nanopatch’s dry-coating technology eliminates the need for vaccine refrigeration during storage and transportation, thereby eliminating the resource burden of maintaining a cold chain.
“Based on our results, we believe that Vaxxas’ HD-MAP could offer a compelling solution that importantly could use less vaccine and potentially could be readily distributed without refrigeration for self-administration,” David A. Muller, Advance Queensland Industry Research Fellow, School of Chemistry and Molecular Biosciences, The University of Queensland, said last June. “This combination could make the HD-MAP extremely well suited to support the massive need for global population vaccination and indeed, we believe that HD-MAP offers a superior alternative to conventional needle-and-syringe.”
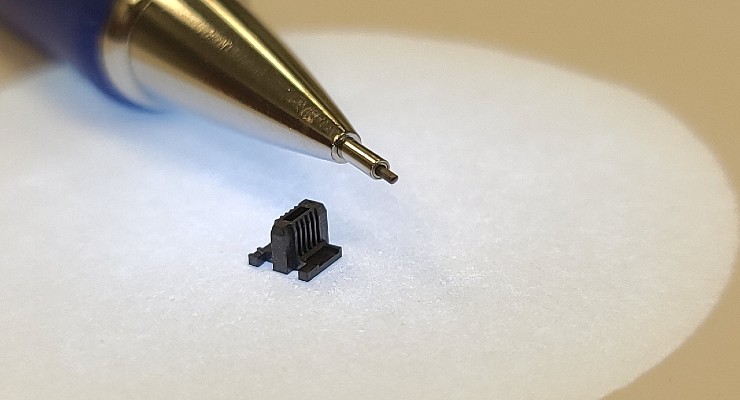
That superiority lies in the microscopic projections responsible for delivering vaccines. Those projections likely were created through micromolding, a highly specialized manufacturing process that produces extremely small, high-precision thermoplastic components with micron tolerances. This technique has become integral to medical device manufacturing of late as devices continue to shrink in size and scale.
MPO’s feature “Big Shots” details the trends and market forces driving micromolding in the medical device industry. Dave Moyak, general manager; and Tom Moyak, director, Business and Engineering, at Matrix Tool Inc., were among the more than one dozen experts interviewed for the feature. Their full input is provided in the following Q&A:
Michael Barbella: What are the latest trends in micromolding technology and services?
Dave Moyak: Part miniaturization is a constant trend in nearly every market segment in industry. In a drive to squeeze as much technology into an ever shrinking footprint, OEMs are looking to suppliers that can help in every phase of the product life cycle. The margin for error is less than razor thin. With so little room for error, a lean manufacturing culture is critical to success in micromolding. I can’t speak for other businesses, but at Matrix we constantly re-evaluate our entire cradle-to-grave process with a focus on continuous improvement and waste reduction. As an engineering-driven company, we are intentional in our actions to simplify our solutions to everyday challenges. We accomplish this through the application of a fundamentally sound engineering approach we call “The Matrix Way.”
Barbella: What are customers demanding or expecting of their micromolded products and have these demands/expectations changed in recent years?
Dave Moyak: Smaller, faster, lighter, better. With traditional plastic parts, the end point is a known destination—using widely known and accepted manufacturing practices. With micro-molded parts, it’s more of a journey. What we know and can accomplish today is a significant percentage better than what we could do five years ago and we’ll say the same thing five years from now. The end point keeps moving because of the advancements in design, tooling, machinery and knowledge. We have over a dozen plastics and mechanical engineers on staff. Matrix Tool typically completes over 100 continuous improvement projects per year. Many of these KPI projects directly improve some aspect of our micro tooling or micromolding capabilities. Innovation and improvement are goalposts that will continue to move by both us and our customers. It’s a never-ending journey.
Barbella: How have advances in materials impacted micromolding technology?
Tom Moyak: As part geometry and wall sections continue to shrink with micro part design, material suppliers are investing in the development of new, easier flowing grades that equal or improve upon the mechanical properties of their predecessors. With wall sections of .003” or less, flow lengths and process capability become real manufacturing challenges. Without the advancements in this area, many of the micro parts on the market today wouldn’t be possible. We partner with multiple material suppliers to trial their experimental grades under real-world challenging applications. Our valuable feedback helps drive material innovations for future micromolding success.
Barbella: Please discuss the challenges and complexities involved in micromolding tooling design. How can these challenges be overcome?
Tom Moyak: It is commonplace in the injection molding industry—if not startlingly surprising—to see how many critical decisions are made, impacting very large sums of money simply because that’s the way things have always been done. For right or wrong, what’s been good enough has sufficed for much of the industry for decades. With such little room for error, micromolding success truly requires the use of fundamentally sound and logical decisions at every step of the process. There has to be a focus on waste reduction through the application of 5S lean manufacturing principles. This is especially true with the foundation of any advanced micromolding part, which is creating a sound tool design.
Some critical aspects of mold design for micromolding include maximizing mold alignment, minimizing mold mass, and minimizing flow lengths with tighter cavity spacing. You must also use creative, balanced layouts, and design the mold to accurately create robust details that can handle the micro-features and micro-gates inherent to the nature of these products. IMMs for micromolding rarely have enough daylight for hot runner systems, nor is the error introduced by these systems helpful towards consistently creating parts of the highest quality. Getting away from old school thinking allows micromolders to realize they may be able to design a mold that is simpler while accomplishing more with less.
One of our recent successes involves convincing a top 10 Fortune company to let us tackle a very high profile program of theirs using “The Matrix Way.” They originally requested that we quote a 16-cavity mold with a hot runner system and other technology for a micro-featured, delicate product. Knowing the material they selected wasn’t optimal and what they requested had a very high probability of failure, we suggested alternative materials. But more importantly we were only willing to quote our completely unique “out of the box” solution. It took some convincing, but our unique eight-cavity all cold-runner solution provided more throughput with less waste than they had estimated for their 16-cavity hot runner solution. The tool we designed and built was simpler and required a much lower capital investment. It had the added bonus of a greatly reduced cycle time and smaller runner sizing while greatly improving part quality and consistency. It was a win-win for all and a real eye opener for our customer, and especially their customer.
Barbella: Design for Manufacturability is critically important in micromolding. How is this different than conventional DfM?
Tom Moyak: To ensure success in micromolding there needs to be a synergy between all phases of the manufacturing process. We can no longer go about each process sequentially—the mold designer doing their part, then the tool builder doing their part, followed by the molder doing their part. At Matrix we create a cross-functional team that stays involved throughout the entire process—we believe that employing the full-service capabilities and expertise under one roof is paramount to our success. Our experience is that DFM for micromolding needs to go one step farther—and earlier. Our highly skilled customers need our product, engineering and materials-related expertise more than ever when it comes to micromolding. The more “say” we’re afforded early in the process increases the likelihood of micromolding success.
Barbella: Is there a limit to how small a micromolded part can be?
Dave Moyak: What is considered an “acceptable flash” specification of .002 -.003” for a conventional molded part is actually the size of many micro part features. Individual micro parts are routinely fractions of a resin pellet. But regardless of the size of the plastic part, successful manufacturing always comes down to three basics: a good tool, a good machine and a good process. Micro molding has continued to stretch the capabilities of the resin suppliers, tool machinery, and injection molding machine OEMs. Equipment manufacturers are constantly creating solutions to the micro paradigm. Many companies, such as Matrix, still have to modify even the best equipment in this field to meet the challenges of the tools we produce and the molds we run. Eventually these modifications become part of the mainstream offerings of the equipment suppliers. Necessity truly drives innovation.
Microsoft, for example, could very well have been the world’s first trillion-dollar company had the public warmed to its tablet at the millennium’s dawning. Similarly, the world was not quite ready to embrace the future when Bell Labs unveiled its Picturephone in 1964 (imagine how different life would be today had it become commonplace back then).
Surprisingly, biotechnology firm Vaxxas hasn’t encountered that same dead end despite its invention being a bit ahead of its own time. The privately-held company is developing needle-free vaccination technology originally hatched by the Australian Institute of Bioengineering & Nanotechnology at The University of Queensland.
The technology uses a proprietary high-density micro-projection array patch (HD-MAP) to streamline and improve vaccine delivery. The postage stamp-sized silicon patch contains tens of thousands of projections 200-300 microns in length that release vaccine antigens directly to immune cells sitting just below the skin’s surface.
Preclinical studies have shown the Nanopatch to be considerably more effective than conventional vaccine delivery systems, with as little as 1/100th of its dose eliciting the same immune response as a “full” portion through intramuscular injection. Moreover, Nanopatch’s dry-coating technology eliminates the need for vaccine refrigeration during storage and transportation, thereby eliminating the resource burden of maintaining a cold chain.
“Based on our results, we believe that Vaxxas’ HD-MAP could offer a compelling solution that importantly could use less vaccine and potentially could be readily distributed without refrigeration for self-administration,” David A. Muller, Advance Queensland Industry Research Fellow, School of Chemistry and Molecular Biosciences, The University of Queensland, said last June. “This combination could make the HD-MAP extremely well suited to support the massive need for global population vaccination and indeed, we believe that HD-MAP offers a superior alternative to conventional needle-and-syringe.”
That superiority lies in the microscopic projections responsible for delivering vaccines. Those projections likely were created through micromolding, a highly specialized manufacturing process that produces extremely small, high-precision thermoplastic components with micron tolerances. This technique has become integral to medical device manufacturing of late as devices continue to shrink in size and scale.
MPO’s feature “Big Shots” details the trends and market forces driving micromolding in the medical device industry. Dave Moyak, general manager; and Tom Moyak, director, Business and Engineering, at Matrix Tool Inc., were among the more than one dozen experts interviewed for the feature. Their full input is provided in the following Q&A:
Michael Barbella: What are the latest trends in micromolding technology and services?
Dave Moyak: Part miniaturization is a constant trend in nearly every market segment in industry. In a drive to squeeze as much technology into an ever shrinking footprint, OEMs are looking to suppliers that can help in every phase of the product life cycle. The margin for error is less than razor thin. With so little room for error, a lean manufacturing culture is critical to success in micromolding. I can’t speak for other businesses, but at Matrix we constantly re-evaluate our entire cradle-to-grave process with a focus on continuous improvement and waste reduction. As an engineering-driven company, we are intentional in our actions to simplify our solutions to everyday challenges. We accomplish this through the application of a fundamentally sound engineering approach we call “The Matrix Way.”
Barbella: What are customers demanding or expecting of their micromolded products and have these demands/expectations changed in recent years?
Dave Moyak: Smaller, faster, lighter, better. With traditional plastic parts, the end point is a known destination—using widely known and accepted manufacturing practices. With micro-molded parts, it’s more of a journey. What we know and can accomplish today is a significant percentage better than what we could do five years ago and we’ll say the same thing five years from now. The end point keeps moving because of the advancements in design, tooling, machinery and knowledge. We have over a dozen plastics and mechanical engineers on staff. Matrix Tool typically completes over 100 continuous improvement projects per year. Many of these KPI projects directly improve some aspect of our micro tooling or micromolding capabilities. Innovation and improvement are goalposts that will continue to move by both us and our customers. It’s a never-ending journey.
Barbella: How have advances in materials impacted micromolding technology?
Tom Moyak: As part geometry and wall sections continue to shrink with micro part design, material suppliers are investing in the development of new, easier flowing grades that equal or improve upon the mechanical properties of their predecessors. With wall sections of .003” or less, flow lengths and process capability become real manufacturing challenges. Without the advancements in this area, many of the micro parts on the market today wouldn’t be possible. We partner with multiple material suppliers to trial their experimental grades under real-world challenging applications. Our valuable feedback helps drive material innovations for future micromolding success.
Barbella: Please discuss the challenges and complexities involved in micromolding tooling design. How can these challenges be overcome?
Tom Moyak: It is commonplace in the injection molding industry—if not startlingly surprising—to see how many critical decisions are made, impacting very large sums of money simply because that’s the way things have always been done. For right or wrong, what’s been good enough has sufficed for much of the industry for decades. With such little room for error, micromolding success truly requires the use of fundamentally sound and logical decisions at every step of the process. There has to be a focus on waste reduction through the application of 5S lean manufacturing principles. This is especially true with the foundation of any advanced micromolding part, which is creating a sound tool design.
Some critical aspects of mold design for micromolding include maximizing mold alignment, minimizing mold mass, and minimizing flow lengths with tighter cavity spacing. You must also use creative, balanced layouts, and design the mold to accurately create robust details that can handle the micro-features and micro-gates inherent to the nature of these products. IMMs for micromolding rarely have enough daylight for hot runner systems, nor is the error introduced by these systems helpful towards consistently creating parts of the highest quality. Getting away from old school thinking allows micromolders to realize they may be able to design a mold that is simpler while accomplishing more with less.
One of our recent successes involves convincing a top 10 Fortune company to let us tackle a very high profile program of theirs using “The Matrix Way.” They originally requested that we quote a 16-cavity mold with a hot runner system and other technology for a micro-featured, delicate product. Knowing the material they selected wasn’t optimal and what they requested had a very high probability of failure, we suggested alternative materials. But more importantly we were only willing to quote our completely unique “out of the box” solution. It took some convincing, but our unique eight-cavity all cold-runner solution provided more throughput with less waste than they had estimated for their 16-cavity hot runner solution. The tool we designed and built was simpler and required a much lower capital investment. It had the added bonus of a greatly reduced cycle time and smaller runner sizing while greatly improving part quality and consistency. It was a win-win for all and a real eye opener for our customer, and especially their customer.
Barbella: Design for Manufacturability is critically important in micromolding. How is this different than conventional DfM?
Tom Moyak: To ensure success in micromolding there needs to be a synergy between all phases of the manufacturing process. We can no longer go about each process sequentially—the mold designer doing their part, then the tool builder doing their part, followed by the molder doing their part. At Matrix we create a cross-functional team that stays involved throughout the entire process—we believe that employing the full-service capabilities and expertise under one roof is paramount to our success. Our experience is that DFM for micromolding needs to go one step farther—and earlier. Our highly skilled customers need our product, engineering and materials-related expertise more than ever when it comes to micromolding. The more “say” we’re afforded early in the process increases the likelihood of micromolding success.
Barbella: Is there a limit to how small a micromolded part can be?
Dave Moyak: What is considered an “acceptable flash” specification of .002 -.003” for a conventional molded part is actually the size of many micro part features. Individual micro parts are routinely fractions of a resin pellet. But regardless of the size of the plastic part, successful manufacturing always comes down to three basics: a good tool, a good machine and a good process. Micro molding has continued to stretch the capabilities of the resin suppliers, tool machinery, and injection molding machine OEMs. Equipment manufacturers are constantly creating solutions to the micro paradigm. Many companies, such as Matrix, still have to modify even the best equipment in this field to meet the challenges of the tools we produce and the molds we run. Eventually these modifications become part of the mainstream offerings of the equipment suppliers. Necessity truly drives innovation.