Louis Columbus, Principal of DELMIAWorks11.09.21
If the past year has reminded us of anything, it is that medical product manufacturing strategies are much like knee replacement implants. No one size fits all.
Instead, manufacturers and the contractors on which they rely need to rapidly adapt and right-size their strategies to changing demands. For example, the 2021 MedTech Contract Manufacturing Report from Alira Health predicts significantly higher growth in the medical device industry, rising from a rate of just 0.9% in 2020 to 11.3% in 2021.
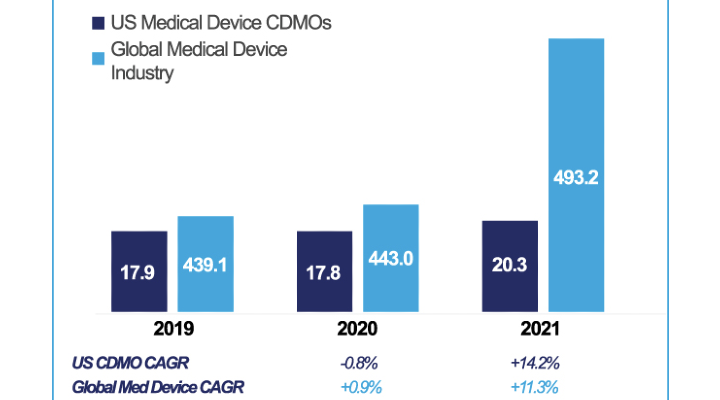
Image courtesy of Alira Health.
At the same time, it is going to be an uneven path to growth and profitability. Medical product companies and manufacturers have seen a reduced need for orthopedic devices due to deferred elective surgeries. On the other hand, the industry is facing spikes in demand for respiratory care products. Meanwhile, shortages of microprocessors and other critical components, combined with delays due to prolonged supply chain issues, add layers of uncertainty.
While some of the current market and supply chain uncertainty are putting new stresses on medical product companies and manufacturers, a longer-term view shows that the ability to adapt to change is a core characteristic for driving market competitiveness and growth. A case in point is recent research on medical technology commercial models from the Boston Consulting Group (BCG). The chart from BCG below shows how medical technology companies that underwent a commercial transformation had a compound annual growth rate (CAGR) of 27% from 2011-2021 versus a 14% CAGR for the S&P 500 over the same time frame.
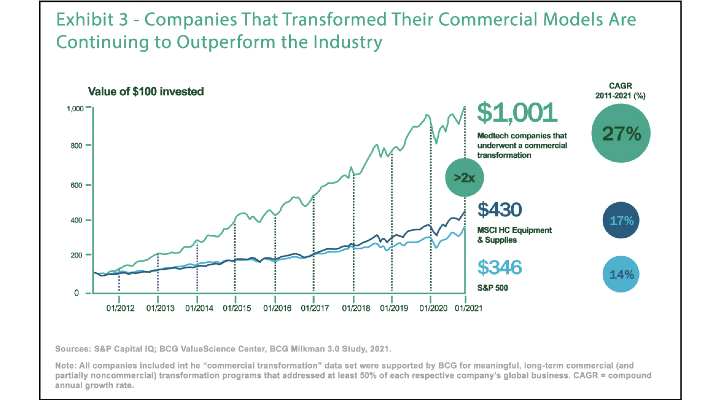
Image courtesy of Boston Consulting Group.
Collectively, these factors are leading many companies to adjust their approaches to expanding operations, managing inventory, and pricing products and components, among other factors. This, in turn, is creating the need for medical product companies and contractors to adopt a more adaptive, contextually intelligent, and flexible approach to shifting from one strategy to another based on the opportunities they find.
Implementing an adaptive manufacturing strategy using manual processes is nearly impossible. To recalibrate their strategies and operations as market conditions change, original equipment manufacturers (OEMs) and contract manufacturers alike need up-to-the-moment data, as well as applications that put this information in context and facilitate collaboration.
Let’s look at the how the technologies already used by many medical product OEMs and contractors can support an adaptive manufacturing strategy, along with five real-world examples of how adaptive manufacturing is being applied today.
Achieving adaptive manufacturing starts with getting CAD designers, product engineers, production planning, manufacturing, and sales teams collaborating in real time together. In many cases, this collaboration needs to extend across teams within the OEM organization and contractor, for example working together on the design of tooling to produce a new implant model or working together to come up with a new pricing model. The flexibility of ERP, MES and CAD/CAM software also plays a critical role in enabling manufacturers to put adaptive strategies into practice.
Consider the North American medical device manufacturer that, working with a leading plastics extrusion producer, took just six weeks to launch an entire series of new ventilator and respiratory products by applying a combination of adaptive manufacturing design-for-manufacturing strategies. By comparison, it would have taken six months or longer to get the same product series launched if the medical device manufacturer had relied on traditional approaches to collaborative new product development.
The plastics extrusion company took CAD files from the medical device OEM and created the needed downstream processes and production-grade models in order to fulfill the ventilator and respiratory product orders that were ramping up from the distribution network. Notably, using information from the CAD files has enabled the plastics extrusion company to produce ventilator and respiratory subassemblies from the OEM’s molds within 72 hours, supporting the need to respond quickly to market demand.
The CRO at the medical product OEM explained, "The current medical device market is so mercurial, we have no choice but to share every aspect of a new product development cycle collaboratively across our team of manufacturing partners. There is no time to lose, and manual approaches are much too slow for how fast this market moves today."
Determining when to switch product lines and then making the change is one example of implementing an adaptive manufacturing strategy. Let’s look at five other common scenarios where medical product companies and manufacturers can apply adaptive manufacturing—making decisions and taking action based on current conditions at any given time.
By contrast, in more constrained situations where there are tight labor markets, higher material prices, and busy equipment, a make-to-order strategy based on advance planning is necessary. Buying materials in advance, perhaps at more favorable prices, along with the precise scheduling of equipment and labor become required steps to ensure the timely production of goods and the ability to fulfill customer demand.
When producing medical products and components, additional factors may come into play. For example, when the microprocessors needed to drive certain devices or instruments are in short supply, the management team will need to re-evaluate and plan for what products will get priority access to these scarce resources. In other words, manufacturers need disciplined planning and forecasting where they are building to forecasts and meeting customer demands from finished goods inventory rather than last-minute production runs.
By using an ERP system’s capabilities for forecasting, scheduling and production planning, materials requirements resource planning, and master production scheduling, manufacturers gain the visibility and control needed to manufacture to forecast.

DELMIAWorks’ Forecasting tools allow users to make informed business decisions.
However, when the market is more volatile, it’s common to see a contractor or supplier have the upper hand in the buyer-seller relationship, and contractors who have the ability to fulfill demand also have a greater ability to dictate price. Not only can they raise prices to maintain margins; they can dedicate production capacity to the customers they choose. By optimizing their pricing and production capacity utilization, these contract manufacturers can capitalize on opportunities to grow the bottom line.
The capabilities within an ERP system to play out what-if scenarios, guide the most profitable production strategies, even automatically adjust pricing as costs rise and fall become powerful multipliers of a contract manufacturer’s opportunity to take full advantage of high demand, low supply macro-economic conditions. Moreover, when capacity utilization, pricing, and contract management systems all share the same data, procurement teams have the knowledge they need to manage fixed-price contracts more profitably.
Currently, construction resources are constrained, and regulatory hurdles for new construction are higher than ever. At the same time, existing properties are broadly available throughout the region, and could be readily modified to add clean rooms. However, most are too distant to manage with the same executive team.
Modern ERP systems make buying existing facilities a viable option. They easily support multiple plant locations and automatically embed established operating procedures into new locations, making it easier to establish and maintain regulatory compliance. Moreover, multi-plant ERP systems provide remote visibility across all locations in a single view. This enables executives to manage large multi-site operations using local management and supervisory level personnel.
By contrast, lights-out or very lightly staffed shifts constitute a leap in operating practices that typically require an investment in automation and manufacturing execution software. However, for products or components that don’t require a full team, such shifts can provide a big payback in labor savings and the burden of staffing and managing a graveyard operation, particularly in seven-days-a-week operations.
ERP systems with native MES capabilities are a natural fit with lights-out operations or lightly staffed shifts. Not only do they have the real-time production and process monitoring capability necessary to maintain production records and visibility in the absence of onsite personnel; they also have the ability to alert management and supervisory staff of issues that arise so corrective actions can be implemented as issues arise during unmanned or minimally staffed periods of operation.

RealTime Production Monitoring puts entire plants on one screen so users can easily monitor the progress and status of each work center.
But in today's more volatile and constrained supply chain, many manufacturers and contractors are negotiating the variability of raw material costs into their production contracts. This strategy comes with the cost of having to document raw materials cost at the lot and batch level. These agreements also pose a raw material deflation risk. If prices drop, the finished good prices have to drop commensurately. This means while prices are on the rise, medical product companies and contract manufacturers are protected, but if they start to drop, companies have to be acutely aware of their raw material inventory cost and its potential impact on revenues.
Managing the link between raw materials cost and finished goods pricing requires a comprehensive inventory control and procurement system operating in conjunction with production planning and execution that is tracked at the batch and lot levels. This allows raw materials costs to be precisely matched to finished goods production pricing, ensuring that profit margins are protected during rising costs, and the revenue expectations are managed during periods of declining material prices.
The key to implementing an adaptive manufacturing strategy is hidden in the data that manufacturing operations produce every day and which teams rely on to communicate and collaborate through their CAD/CAM, PLM, ERP and MES software. Parsing and analyzing this data in new ways will enable medical product companies and contract manufacturers to make strategic decisions, adjust their production, and update their policies to align with current market conditions.
Importantly, when medical product OEMs and contract manufacturers rely on an adaptive manufacturing strategy, change is no longer a roadblock; it’s a path to higher profitability and growth.
References:
The 2021 MedTech Contract Manufacturing Report, Alira Health in collaboration with Life Science Intelligence and MassMEDIC, https://alirahealth.com/education-hub/medtechreport2021/, March 2021
The Rise of the Next Generation of Medtech "Milkmen". Boston Consulting Group. https://www.bcg.com/publications/2021/six-design-strategies-for-medical-technology-next-generation-commercial-model. June 16, 2021
Louis Columbus is currently serving as principal of DELMIAWorks. Previous positions include product management at Ingram Cloud, product marketing at iBASEt, Plex Systems, senior analyst at AMR Research (now Gartner), marketing and business development at Cincom Systems, Ingram Micro, a SaaS start-up and at hardware companies. He’s also a member of the Enterprise Irregulars. Professional experience includes marketing, product management, sales and industry analyst roles in the enterprise software and IT industries. Columbus’ academic background includes an MBA from Pepperdine University and completion of the Strategic Marketing Management and Digital Marketing Programs at the Stanford University Graduate School of Business. He teaches MBA courses in international business, global competitive strategies, international market research, and capstone courses in strategic planning and market research. Columbus has taught at California State University, Fullerton: University of California, Irvine; Marymount University, and Webster University.
You can reach him on Twitter at @LouisColumbus.
Instead, manufacturers and the contractors on which they rely need to rapidly adapt and right-size their strategies to changing demands. For example, the 2021 MedTech Contract Manufacturing Report from Alira Health predicts significantly higher growth in the medical device industry, rising from a rate of just 0.9% in 2020 to 11.3% in 2021.

Image courtesy of Alira Health.
At the same time, it is going to be an uneven path to growth and profitability. Medical product companies and manufacturers have seen a reduced need for orthopedic devices due to deferred elective surgeries. On the other hand, the industry is facing spikes in demand for respiratory care products. Meanwhile, shortages of microprocessors and other critical components, combined with delays due to prolonged supply chain issues, add layers of uncertainty.
While some of the current market and supply chain uncertainty are putting new stresses on medical product companies and manufacturers, a longer-term view shows that the ability to adapt to change is a core characteristic for driving market competitiveness and growth. A case in point is recent research on medical technology commercial models from the Boston Consulting Group (BCG). The chart from BCG below shows how medical technology companies that underwent a commercial transformation had a compound annual growth rate (CAGR) of 27% from 2011-2021 versus a 14% CAGR for the S&P 500 over the same time frame.

Image courtesy of Boston Consulting Group.
Collectively, these factors are leading many companies to adjust their approaches to expanding operations, managing inventory, and pricing products and components, among other factors. This, in turn, is creating the need for medical product companies and contractors to adopt a more adaptive, contextually intelligent, and flexible approach to shifting from one strategy to another based on the opportunities they find.
Implementing an adaptive manufacturing strategy using manual processes is nearly impossible. To recalibrate their strategies and operations as market conditions change, original equipment manufacturers (OEMs) and contract manufacturers alike need up-to-the-moment data, as well as applications that put this information in context and facilitate collaboration.
Let’s look at the how the technologies already used by many medical product OEMs and contractors can support an adaptive manufacturing strategy, along with five real-world examples of how adaptive manufacturing is being applied today.
How Adaptive Manufacturing Strategies Work
Adaptive manufacturing is predicated on the understanding that every automated system used by a medical product OEM or contractor manufacturer runs at a different clock speed. Computer-aided design and manufacturing (CAD/CAM), product lifecycle management (PLM), enterprise resource planning (ERP), and manufacturing execution system (MES) solutions have been purpose-built for specific functions and therefore naturally run at various speeds. Only when all systems are synchronized to the same cadence can an entire production center effectively adapt.Achieving adaptive manufacturing starts with getting CAD designers, product engineers, production planning, manufacturing, and sales teams collaborating in real time together. In many cases, this collaboration needs to extend across teams within the OEM organization and contractor, for example working together on the design of tooling to produce a new implant model or working together to come up with a new pricing model. The flexibility of ERP, MES and CAD/CAM software also plays a critical role in enabling manufacturers to put adaptive strategies into practice.
Consider the North American medical device manufacturer that, working with a leading plastics extrusion producer, took just six weeks to launch an entire series of new ventilator and respiratory products by applying a combination of adaptive manufacturing design-for-manufacturing strategies. By comparison, it would have taken six months or longer to get the same product series launched if the medical device manufacturer had relied on traditional approaches to collaborative new product development.
The plastics extrusion company took CAD files from the medical device OEM and created the needed downstream processes and production-grade models in order to fulfill the ventilator and respiratory product orders that were ramping up from the distribution network. Notably, using information from the CAD files has enabled the plastics extrusion company to produce ventilator and respiratory subassemblies from the OEM’s molds within 72 hours, supporting the need to respond quickly to market demand.
The CRO at the medical product OEM explained, "The current medical device market is so mercurial, we have no choice but to share every aspect of a new product development cycle collaboratively across our team of manufacturing partners. There is no time to lose, and manual approaches are much too slow for how fast this market moves today."
Determining when to switch product lines and then making the change is one example of implementing an adaptive manufacturing strategy. Let’s look at five other common scenarios where medical product companies and manufacturers can apply adaptive manufacturing—making decisions and taking action based on current conditions at any given time.
Reconsidering Just-in-Time Manufacturing
Just-in-time manufacturing is often a winning strategy in periods of plentiful raw materials, equipment, and labor. The lack of scheduling constraints makes last-minute production fairly straightforward and matches expenses very closely with revenue. Here, ERP and MES systems facilitate the switches between production runs to meet demand. For example, one medical device manufacturer’s integrated ERP and MES software can flex between 28 different production workflows, which makes reconfiguring production lines for engineer-to-order products efficient with minimal disruptions to production.By contrast, in more constrained situations where there are tight labor markets, higher material prices, and busy equipment, a make-to-order strategy based on advance planning is necessary. Buying materials in advance, perhaps at more favorable prices, along with the precise scheduling of equipment and labor become required steps to ensure the timely production of goods and the ability to fulfill customer demand.
When producing medical products and components, additional factors may come into play. For example, when the microprocessors needed to drive certain devices or instruments are in short supply, the management team will need to re-evaluate and plan for what products will get priority access to these scarce resources. In other words, manufacturers need disciplined planning and forecasting where they are building to forecasts and meeting customer demands from finished goods inventory rather than last-minute production runs.
By using an ERP system’s capabilities for forecasting, scheduling and production planning, materials requirements resource planning, and master production scheduling, manufacturers gain the visibility and control needed to manufacture to forecast.

DELMIAWorks’ Forecasting tools allow users to make informed business decisions.
Weighing Pricing Strategies for Contracts
In many situations, long-term, fixed-price contracts are attractive because they can ensure revenue flow and capacity utilization. During stable economic and market periods, long-term agreements are also necessary for winning business, since buyers are looking for predictability with both supplies and costs.However, when the market is more volatile, it’s common to see a contractor or supplier have the upper hand in the buyer-seller relationship, and contractors who have the ability to fulfill demand also have a greater ability to dictate price. Not only can they raise prices to maintain margins; they can dedicate production capacity to the customers they choose. By optimizing their pricing and production capacity utilization, these contract manufacturers can capitalize on opportunities to grow the bottom line.
The capabilities within an ERP system to play out what-if scenarios, guide the most profitable production strategies, even automatically adjust pricing as costs rise and fall become powerful multipliers of a contract manufacturer’s opportunity to take full advantage of high demand, low supply macro-economic conditions. Moreover, when capacity utilization, pricing, and contract management systems all share the same data, procurement teams have the knowledge they need to manage fixed-price contracts more profitably.
Adding Plant Capacity: Build Vs. Buy
In the past, a medical product OEM or contract manufacturer would typically build additional plant capacity on adjacent properties in the local business park. The resources to build were readily available, and the proximity made management oversight of the additional capacity fairly straightforward.Currently, construction resources are constrained, and regulatory hurdles for new construction are higher than ever. At the same time, existing properties are broadly available throughout the region, and could be readily modified to add clean rooms. However, most are too distant to manage with the same executive team.
Modern ERP systems make buying existing facilities a viable option. They easily support multiple plant locations and automatically embed established operating procedures into new locations, making it easier to establish and maintain regulatory compliance. Moreover, multi-plant ERP systems provide remote visibility across all locations in a single view. This enables executives to manage large multi-site operations using local management and supervisory level personnel.
Bringing on a Third Shift: Staffing Strategies
Running a manned third shift is not easy, but it is a straightforward extension of established operating practices, and in the case of some medical products, it is an absolute necessity. Staffing extra shifts is more difficult because of labor shortages, but it is still, a linear adjustment compared to a leap in operating practices.By contrast, lights-out or very lightly staffed shifts constitute a leap in operating practices that typically require an investment in automation and manufacturing execution software. However, for products or components that don’t require a full team, such shifts can provide a big payback in labor savings and the burden of staffing and managing a graveyard operation, particularly in seven-days-a-week operations.
ERP systems with native MES capabilities are a natural fit with lights-out operations or lightly staffed shifts. Not only do they have the real-time production and process monitoring capability necessary to maintain production records and visibility in the absence of onsite personnel; they also have the ability to alert management and supervisory staff of issues that arise so corrective actions can be implemented as issues arise during unmanned or minimally staffed periods of operation.

RealTime Production Monitoring puts entire plants on one screen so users can easily monitor the progress and status of each work center.
Linking Finished Goods and Raw Material Pricing
In stable supply situations, most medical product companies and contract manufacturers prefer not to link finished goods prices to raw material costs contractually. Most would rather rely on their buying savvy and procurement management skills to meet or perhaps even undercut original raw materials cost estimates.But in today's more volatile and constrained supply chain, many manufacturers and contractors are negotiating the variability of raw material costs into their production contracts. This strategy comes with the cost of having to document raw materials cost at the lot and batch level. These agreements also pose a raw material deflation risk. If prices drop, the finished good prices have to drop commensurately. This means while prices are on the rise, medical product companies and contract manufacturers are protected, but if they start to drop, companies have to be acutely aware of their raw material inventory cost and its potential impact on revenues.
Managing the link between raw materials cost and finished goods pricing requires a comprehensive inventory control and procurement system operating in conjunction with production planning and execution that is tracked at the batch and lot levels. This allows raw materials costs to be precisely matched to finished goods production pricing, ensuring that profit margins are protected during rising costs, and the revenue expectations are managed during periods of declining material prices.
Conclusion
Years of relative market stability have led many of us to take certain manufacturing strategies as ultimate truths, whether it is just-in-time manufacturing or fixed-price contracts. However, the rapid spikes and drops in market demand, shortages in supplies and raw materials, and supply chain uncertainty have highlighted the need for medical product OEMs and contract manufacturers to adjust their approaches as market and economic conditions evolve. In short, they need to embrace an adaptive manufacturing strategy.The key to implementing an adaptive manufacturing strategy is hidden in the data that manufacturing operations produce every day and which teams rely on to communicate and collaborate through their CAD/CAM, PLM, ERP and MES software. Parsing and analyzing this data in new ways will enable medical product companies and contract manufacturers to make strategic decisions, adjust their production, and update their policies to align with current market conditions.
Importantly, when medical product OEMs and contract manufacturers rely on an adaptive manufacturing strategy, change is no longer a roadblock; it’s a path to higher profitability and growth.
References:
The 2021 MedTech Contract Manufacturing Report, Alira Health in collaboration with Life Science Intelligence and MassMEDIC, https://alirahealth.com/education-hub/medtechreport2021/, March 2021
The Rise of the Next Generation of Medtech "Milkmen". Boston Consulting Group. https://www.bcg.com/publications/2021/six-design-strategies-for-medical-technology-next-generation-commercial-model. June 16, 2021
Louis Columbus is currently serving as principal of DELMIAWorks. Previous positions include product management at Ingram Cloud, product marketing at iBASEt, Plex Systems, senior analyst at AMR Research (now Gartner), marketing and business development at Cincom Systems, Ingram Micro, a SaaS start-up and at hardware companies. He’s also a member of the Enterprise Irregulars. Professional experience includes marketing, product management, sales and industry analyst roles in the enterprise software and IT industries. Columbus’ academic background includes an MBA from Pepperdine University and completion of the Strategic Marketing Management and Digital Marketing Programs at the Stanford University Graduate School of Business. He teaches MBA courses in international business, global competitive strategies, international market research, and capstone courses in strategic planning and market research. Columbus has taught at California State University, Fullerton: University of California, Irvine; Marymount University, and Webster University.
You can reach him on Twitter at @LouisColumbus.












