Adrian Possumato, Principal Consultant, Medica Consulting05.21.19
Unlike most scale-up efforts where the simple maintenance of quality is paramount, dms-service challenged Comar to make significant process improvements in an effort to increase their already high level of quality without disrupting supply. The principal focus of this effort was the recorder’s hermetic seal achieved via ultrasonic welding during assembly of the molded recorder components.
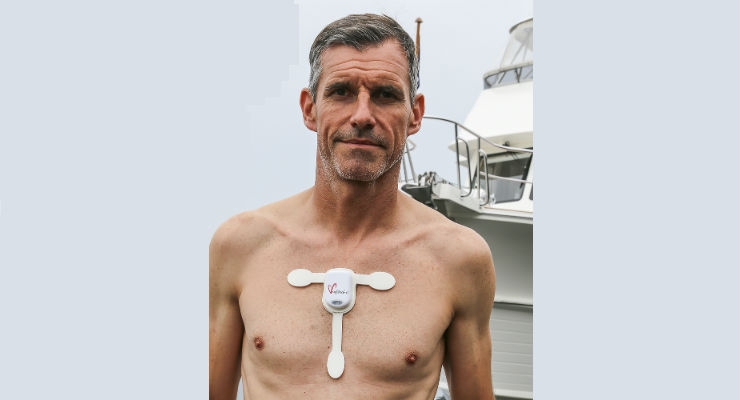
Ambulatory ECG Monitor
Lynda Cole knew she would need additional manufacturing capacity to support the successful 2017 launch of dms-service’s innovative myPatch sl Holter recorder.
Cole, owner and manager of dms-service, first started in the Holter recorder industry in 1977 with her father’s business, Scole Engineering Co. Inc. Since that time, she has witnessed first-hand the technological advances in Holter monitoring that are represented in the revolutionary myPatch sl design.
These technical advances have resulted in a recorder design that requires advanced molding and assembly capabilities.
As an ambulatory electrocardiogram (ECG) monitor, the myPatch sl recorder is regulated by the United States Food and Drug Administration (FDA) as a Class II medical device under 21 CFR Part 820 and supported by a 510(k) regulatory clearance by FDA’s Center for Devices and Radiological Health.
Waterproof Design
dms-service released a truly innovative solution with the myPatch sl recorder. The myPatch sl features an IP rating of 68—waterproof up to two meters of water for one hour and two channels of continuous ECG recording for 14 days. It offers multiple electrode sizes for patient comfort and convenience, and is available in adult, pediatric, ladies, sports, and neonate electrodes.
What does this IP rating mean? It means the patient does not need to worry about taking a shower while wearing the device or a quick swim with the sports electrode.
To achieve this IP rating and prevent recorder failure, the myPatch sl design relies on a hermetic, ultrasonic seal between the injection molded recorder housing components and the button overmold to protect the recorder’s internal PC circuit board and battery.
Choosing a Supplier
“The advanced design of the myPatch sl recorder coupled with the quality systems and regulatory compliance requirements to support an FDA cleared medical device also limited dms-service's choices for an alternative molder/assembler,” cited Cole. “We needed a supplier that offered the full suite of services, as well as, the agility required to support the molding and assembly of our device."
Comar was selected as it offered ISO 13485:2016 and ISO 9001:2015 facilities, Class 8 cleanrooms, and established FDA registration for medical devices. The manufacturer’s capabilities included design-for-manufacturing services, in-house mold making and prototyping, and diverse molding technologies with a strict QA/RA umbrella.
“We recognize the benefit that agility plays in delivering a quality product to market quickly,” stated Scott Conklin, Comar’s executive vice president of sales & marketing. “Comar has provided medical device and diagnostic manufacturers this benefit for a number of years now. The market rewards the first to arrive and it is incumbent on Comar to serve as a partner to our customers.”
Improving Quality During Scale-Up
“Improving on an already high IP score against water ingress was a challenge during scale-up operations,” explained Scott Taylor, medical device market director for Comar. “We have very strong resources and capabilities in design, manufacturing, and final testing whereby we were able to easily modify the recorder joint design and introduce an improved ultrasonic welding horn, improving output and quality simultaneously.”
Losses/scrap during the ultrasonic welding operation were reduced from 22 percent to less than 2 percent and further improvements continue.
Similarly, design-for-manufacturing improvements were made to the insert molding of the electrical contacts on the underside of the recorder and overmolding of the button on its topside. Specifically, positioning and retention of the contacts and snaps placed in the mold prior to the injection were improved, thus making it easier for the machine operator. Since it was made easier to do it the same every time, the overall product consistency improved dramatically.
“It was critical that supply chain not be adversely affected during the implementation of these improvements.” said Cole. “Our market growth plans could not tolerate any delay. I was very impressed with Comar’s capabilities as all of the design and manufacturing improvements made not only kept our commercial plans on track, they were successfully and concurrently implemented. Comar has helped us considerably.”
Moving Forward
“We are very pleased to be entrusted by dms-service to assist with the growth of their business. We remain committed to meeting the needs of our medical device and pharmaceutical customers and are strategically positioned to support these industries,” explained Mike Ruggieri, CEO of Comar.
Ben Lecomte Takes the Plunge
What can happen to the human heart after months of swimming? Ben Lecomte’s historic 5,500-mile swim from Japan to San Francisco is a feat that can’t be missed. Watch the video below to learn about how the myPatch sl recorder is being used to measure the impact of the long-distance swim on his heart.
Adrian Possumato is a principal consultant at Medica Consulting, a boutique pharmaceutical and medical device industry consultant.
Ambulatory ECG Monitor
Lynda Cole knew she would need additional manufacturing capacity to support the successful 2017 launch of dms-service’s innovative myPatch sl Holter recorder.
Cole, owner and manager of dms-service, first started in the Holter recorder industry in 1977 with her father’s business, Scole Engineering Co. Inc. Since that time, she has witnessed first-hand the technological advances in Holter monitoring that are represented in the revolutionary myPatch sl design.
These technical advances have resulted in a recorder design that requires advanced molding and assembly capabilities.
As an ambulatory electrocardiogram (ECG) monitor, the myPatch sl recorder is regulated by the United States Food and Drug Administration (FDA) as a Class II medical device under 21 CFR Part 820 and supported by a 510(k) regulatory clearance by FDA’s Center for Devices and Radiological Health.
Waterproof Design
dms-service released a truly innovative solution with the myPatch sl recorder. The myPatch sl features an IP rating of 68—waterproof up to two meters of water for one hour and two channels of continuous ECG recording for 14 days. It offers multiple electrode sizes for patient comfort and convenience, and is available in adult, pediatric, ladies, sports, and neonate electrodes.
What does this IP rating mean? It means the patient does not need to worry about taking a shower while wearing the device or a quick swim with the sports electrode.
To achieve this IP rating and prevent recorder failure, the myPatch sl design relies on a hermetic, ultrasonic seal between the injection molded recorder housing components and the button overmold to protect the recorder’s internal PC circuit board and battery.
Choosing a Supplier
“The advanced design of the myPatch sl recorder coupled with the quality systems and regulatory compliance requirements to support an FDA cleared medical device also limited dms-service's choices for an alternative molder/assembler,” cited Cole. “We needed a supplier that offered the full suite of services, as well as, the agility required to support the molding and assembly of our device."
Comar was selected as it offered ISO 13485:2016 and ISO 9001:2015 facilities, Class 8 cleanrooms, and established FDA registration for medical devices. The manufacturer’s capabilities included design-for-manufacturing services, in-house mold making and prototyping, and diverse molding technologies with a strict QA/RA umbrella.
“We recognize the benefit that agility plays in delivering a quality product to market quickly,” stated Scott Conklin, Comar’s executive vice president of sales & marketing. “Comar has provided medical device and diagnostic manufacturers this benefit for a number of years now. The market rewards the first to arrive and it is incumbent on Comar to serve as a partner to our customers.”
Improving Quality During Scale-Up
“Improving on an already high IP score against water ingress was a challenge during scale-up operations,” explained Scott Taylor, medical device market director for Comar. “We have very strong resources and capabilities in design, manufacturing, and final testing whereby we were able to easily modify the recorder joint design and introduce an improved ultrasonic welding horn, improving output and quality simultaneously.”
Losses/scrap during the ultrasonic welding operation were reduced from 22 percent to less than 2 percent and further improvements continue.
Similarly, design-for-manufacturing improvements were made to the insert molding of the electrical contacts on the underside of the recorder and overmolding of the button on its topside. Specifically, positioning and retention of the contacts and snaps placed in the mold prior to the injection were improved, thus making it easier for the machine operator. Since it was made easier to do it the same every time, the overall product consistency improved dramatically.
“It was critical that supply chain not be adversely affected during the implementation of these improvements.” said Cole. “Our market growth plans could not tolerate any delay. I was very impressed with Comar’s capabilities as all of the design and manufacturing improvements made not only kept our commercial plans on track, they were successfully and concurrently implemented. Comar has helped us considerably.”
Moving Forward
“We are very pleased to be entrusted by dms-service to assist with the growth of their business. We remain committed to meeting the needs of our medical device and pharmaceutical customers and are strategically positioned to support these industries,” explained Mike Ruggieri, CEO of Comar.
Ben Lecomte Takes the Plunge
What can happen to the human heart after months of swimming? Ben Lecomte’s historic 5,500-mile swim from Japan to San Francisco is a feat that can’t be missed. Watch the video below to learn about how the myPatch sl recorder is being used to measure the impact of the long-distance swim on his heart.
Adrian Possumato is a principal consultant at Medica Consulting, a boutique pharmaceutical and medical device industry consultant.