Jeremy Williams, Consultant/Trainer—TZERO, RJG02.28.19
“Just make the part to print, that’s all.”
We’ve all heard this statement before, right? But it’s not that simple. Engineering a quality part, mold, and process requires a significant understanding of all the plastic and machine variables.
Though there are many strategies to processing plastics, RJG has developed three methods that are each effective in different scenarios—Decoupled Molding I, II, and III. It’s important to review each method to ensure the right one is selected for the correct application.
Let’s take a sample part and review the part design, material selection, mold design, and real processing data for each method to determine how using Decoupled Molding I, II, or III affects the outcome of the part and which method would be the most effective in this application.
Part Design
For the purposes of this article, we are using an ASTM tensile test bar D638 V (Figure 1).
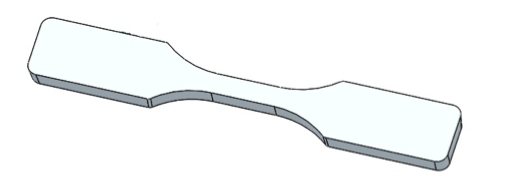
Figure 1: ASTM Tensile Test Bar
The tensile test bar is used to generate many of the values that are found on a technical data sheet. In the next section, we will focus on several of those values.
Material Selection
There are thousands of materials available on the market, but for now, we’ll follow the K.I.S.S. (keep it simple, stupid) principle. However, to ensure we capture a vast portion of the industry, our selections need to cover both amorphous and semi-crystalline materials. Let’s use Toyolac 100 (ABS) and Exxon AX03BE3 (PP).
Heat Deflection/Distortion Temperature (HDT) is the temperature at which a material will deflect under load. Mold designers and simulation users apply this value when designing the cooling channels for the mold. Molders use this value as a target to ensure the part is rigid enough to not get white stress marks, pin push, or (if you’re really lucky) pierce the part with the ejector pins because the part is too soft. As hot plastic gives up its heat, the molecules want to contract back to their original relaxed state—this is commonly called shrink rate.
Mold Design
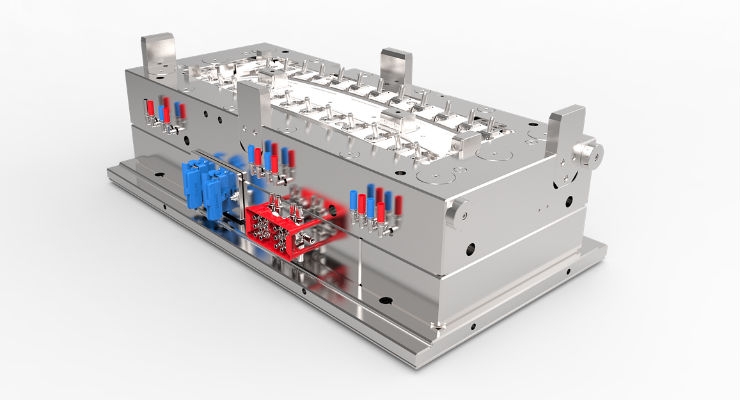
The mold used for testing purposes is an 8-cavity H pattern cold runner with lapped edge gates built by Extreme Tool and Engineering (Figure 2). The mold is instrumented with RJG’s multi-channel button pressure sensors and flush mount temperature sensors to allow us to see inside the cavity and correlate data with molded parts.

Figure 2: Ejector half of mold used for testing
The cavity length is 2.48 inches, width is 0.369 inches, and thickness is 0.133 inches.
Processing
Decoupled Molding is how the process separates the fill, pack, and hold phases. Each has its own purpose, but we will get to that momentarily.
We must recall that the molding machine can control the injection of material with speed or pressure as the controlled variable. The controlled variable depends upon how we segregate the phases and which other variables are driving the proverbial bus.
Decoupled I is essentially a drag race to get material to the end of the cavity as fast as possible. It is most often used for thin-wall molding, which is typically considered anything under 0.06 in. That’s because thin wall parts freeze very quickly, and in order to fill the cavity, we must inject as fast as possible with very high pressure to prevent short shots. Here filling/packing/holding is controlled by injection velocity because the material slows down and the flow front freezes, yielding a short shot.
When using the industry standard Decoupled II, the filling is controlled by velocity, while pack and hold are controlled by pressure.
For critical parts, Decoupled III is typically selected. Filling is a set, fast velocity (often referred to as V1), packing is a slower, controlled velocity (often referred to as V2), and holding is a fixed pressure setpoint.
To ensure consistency across all processing strategies for this test, an end of cavity of 3,000 PSIp was maintained.
Table 2 presents the actual temperatures for the established robust Decoupled II process.
To ensure the part is below the HDT, we utilize thermal imaging to capture all cavities (Figure 3). Thermal management is critical to maintain part quality over time.

Figure 3: Part Temperature at Ejection for ABS
Data, Data, Data, and More Data
For continuity between all data, we chose to use cavity 8 for all measurements because it will have the highest variation due to shear imbalances. This cavity is located in the lower left corner of Figure 4.
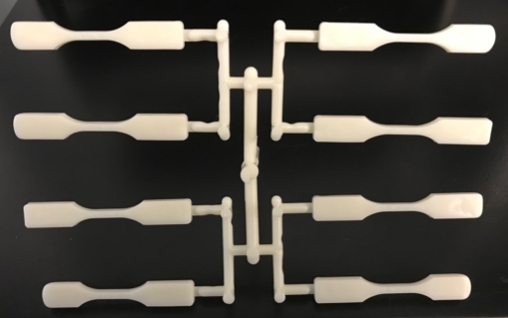
Figure 4: ABS Decoupled II Fill Only
The cavity overall length (OAL) is 2.48 inches so all dimensional data will be based against this value.
Dimensional
A Decoupled I process shows the largest shrink rate. This is because the wall freezes very quickly and does not allow for typical packing that compensates for shrinkage. Based on the material guidelines, the actual shrink rate is outside those limits by 0.0022 in./in. for the ABS. In comparison, the PP falls within the range near the center of the supplied range. When reviewing the changes in OAL, it has a range of 0.0015 inches.
Now let’s review how the material performed utilizing a Decoupled II strategy. For the ABS, the shrink rate is smaller but still outside the range provided by the material supplier by 0.0002 in/in. The same trend for shrink rate is observed in PP, but now is 0.0015 in./in. above the minimum shrinkage expected. With both materials, there is a reduction in OAL range of measurements from 0.0015 inches to 0.001 inches. The average length for the ABS was 0.005 inches longer, while the PP grew 0.0142 inches compared to its Decoupled I counterpart.
Onward to explore the results from the Decoupled III process. For both resins, the shrink rate is nearly identical, but the range in part OAL is reduced from 0.001 inches to 0.0005 inches compared to a Decoupled II process. If we review the part weight changes in PP and ABS, they are alike. This process has the tightest range for two reasons. First, both filling and packing phases are velocity controlled, allowing the machine to use the pressure needed to maintain the set velocity. This allows for two of three phases to compensate for changes in the material automatically by the machine. The second reason is the control of transfer is managed by the cavity pressure sensor not the screw position on the molding machine.
Part Weight
For a Decoupled I process, the trend was essentially the difference between amorphous or semi-crystalline resins with weight varying 0.002 grams. One item we must take into account is that this part would likely never be molded using this process strategy as it’s not considered thin wall.
In a Decoupled II, part weight for both materials show an increase. For ABS, it’s 0.07 grams and 0.13 grams (Figure 5). These changes occur because of the pack/hold phase using a constant pressure to cram more polymer chains into the cavity to compensate for naturally occurring shrinkage that occurs during cooling.

Figure 5: Left ABS, Right PP weight change in pellets
The weight gain shifting to a Decoupled III process is 0.0008 grams for ABS and 0.0002 grams for PP.
Conclusion
In short, a Decoupled I process is best used for thin wall molding applications, Decoupled III is best for high tolerance molding, and Decoupled II is the industry standard for the majority of molded parts.
Trying to validate a part with the tight tolerances of ±0.002 inches and Cpk of 1.67 is going to be nearly impossible as the variation in OAL is using nearly the total tolerance band. The tried and true Decoupled II process will work for most projects as it produces parts with a range in OAL of 0.001 inches and a tolerance of ±0.002 inches, which would allow us to validate with a Cpk of 1.33, but not 1.67. The Decoupled III has a variation in OAL of 0.0005 inches, allowing a tighter tolerance of ±0.001 inches and a Cpk of 1.33.
Keep in mind, this is a very simple part with a single gate. As the project becomes more complex, choosing the correct process strategy is critical so the appropriate shrink rate can be cut into the mold cavity. This isn’t easy, but if it was, everyone would do it. Sit down as a team of part designers, mold designers, and molders and have healthy discussions so the project can be on time, under budget, and right the first time.
Nothing is impossible, we just need to do our due diligence as engineers.
Jeremy Williams has over 20 years of experience in the plastics industry serving the medical, automotive, furniture, and appliance industries. He previously worked as a Principal Engineer, taking projects from design concept to saleable products. Jeremy earned his Master Molder II certification in 2011, became an RJG Certified Trainer in 2012, and started at RJG in 2015. In addition to his extensive manufacturing background, he holds degrees in plastics and business. Currently Jeremy is a Consultant/Trainer with TZERO.
We’ve all heard this statement before, right? But it’s not that simple. Engineering a quality part, mold, and process requires a significant understanding of all the plastic and machine variables.
Though there are many strategies to processing plastics, RJG has developed three methods that are each effective in different scenarios—Decoupled Molding I, II, and III. It’s important to review each method to ensure the right one is selected for the correct application.
Let’s take a sample part and review the part design, material selection, mold design, and real processing data for each method to determine how using Decoupled Molding I, II, or III affects the outcome of the part and which method would be the most effective in this application.
Part Design
For the purposes of this article, we are using an ASTM tensile test bar D638 V (Figure 1).

Figure 1: ASTM Tensile Test Bar
The tensile test bar is used to generate many of the values that are found on a technical data sheet. In the next section, we will focus on several of those values.
Material Selection
There are thousands of materials available on the market, but for now, we’ll follow the K.I.S.S. (keep it simple, stupid) principle. However, to ensure we capture a vast portion of the industry, our selections need to cover both amorphous and semi-crystalline materials. Let’s use Toyolac 100 (ABS) and Exxon AX03BE3 (PP).
| Toyolac 100 | Exxon AX03BE3 | |
| MFI g/10 min | 15.0 | 35.0 |
| Melt °F | 446 to 482 | 392 to 572 |
| Mold °F | 104 to 176 | 60 to 150 |
| HDT °F | 181 | 131 |
| Shrink Rate in./in. | 0.004 to 0.006 | 0.010 to 0.025 |
Table 1: ABS vs PP
Heat Deflection/Distortion Temperature (HDT) is the temperature at which a material will deflect under load. Mold designers and simulation users apply this value when designing the cooling channels for the mold. Molders use this value as a target to ensure the part is rigid enough to not get white stress marks, pin push, or (if you’re really lucky) pierce the part with the ejector pins because the part is too soft. As hot plastic gives up its heat, the molecules want to contract back to their original relaxed state—this is commonly called shrink rate.
Mold Design
The mold used for testing purposes is an 8-cavity H pattern cold runner with lapped edge gates built by Extreme Tool and Engineering (Figure 2). The mold is instrumented with RJG’s multi-channel button pressure sensors and flush mount temperature sensors to allow us to see inside the cavity and correlate data with molded parts.

Figure 2: Ejector half of mold used for testing
The cavity length is 2.48 inches, width is 0.369 inches, and thickness is 0.133 inches.
Processing
Decoupled Molding is how the process separates the fill, pack, and hold phases. Each has its own purpose, but we will get to that momentarily.
We must recall that the molding machine can control the injection of material with speed or pressure as the controlled variable. The controlled variable depends upon how we segregate the phases and which other variables are driving the proverbial bus.
Decoupled I is essentially a drag race to get material to the end of the cavity as fast as possible. It is most often used for thin-wall molding, which is typically considered anything under 0.06 in. That’s because thin wall parts freeze very quickly, and in order to fill the cavity, we must inject as fast as possible with very high pressure to prevent short shots. Here filling/packing/holding is controlled by injection velocity because the material slows down and the flow front freezes, yielding a short shot.
When using the industry standard Decoupled II, the filling is controlled by velocity, while pack and hold are controlled by pressure.
For critical parts, Decoupled III is typically selected. Filling is a set, fast velocity (often referred to as V1), packing is a slower, controlled velocity (often referred to as V2), and holding is a fixed pressure setpoint.
To ensure consistency across all processing strategies for this test, an end of cavity of 3,000 PSIp was maintained.
Table 2 presents the actual temperatures for the established robust Decoupled II process.
| Toyolac 100 | Exxon AX03BE3 | |
| Melt Temperature | 449 | 435 |
| Mold °F | 131 | 72 |
| Ejected Temperature °F | 176 | 123 |
Table 2: Process Temperatures
To ensure the part is below the HDT, we utilize thermal imaging to capture all cavities (Figure 3). Thermal management is critical to maintain part quality over time.

Figure 3: Part Temperature at Ejection for ABS
Data, Data, Data, and More Data
For continuity between all data, we chose to use cavity 8 for all measurements because it will have the highest variation due to shear imbalances. This cavity is located in the lower left corner of Figure 4.

Figure 4: ABS Decoupled II Fill Only
The cavity overall length (OAL) is 2.48 inches so all dimensional data will be based against this value.
Dimensional
| Toyolac 100 Part Size (in) | |||
| DI | DII | DIII | |
| 1 | 2.4600 | 2.4650 | 2.4645 |
| 2 | 2.4595 | 2.4650 | 2.4640 |
| 3 | 2.4595 | 2.4650 | 2.4640 |
| 4 | 2.4595 | 2.4640 | 2.4640 |
| 5 | 2.4585 | 2.4640 | 2.4640 |
| 6 | 2.4590 | 2.4650 | 2.4640 |
| 7 | 2.4600 | 2.4645 | 2.4640 |
| 8 | 2.4600 | 2.4640 | 2.4640 |
| 9 | 2.4600 | 2.4645 | 2.4640 |
| 10 | 2.4595 | 2.4645 | 2.4640 |
| High | 2.4600 | 2.4650 | 2.4645 |
| Average | 2.4596 | 2.4646 | 2.4641 |
| Low | 2.4585 | 2.4640 | 2.4640 |
| Range | 0.0015 | 0.0010 | 0.0005 |
| Standard Deviation | 0.0005 | 0.0004 | 0.0002 |
| Shrink Rate (in./in.) | |||
| Maximum | 0.0087 | 0.0065 | 0.0065 |
| Average | 0.0082 | 0.0062 | 0.0064 |
| Minimum | 0.0081 | 0.0060 | 0.0062 |
| Range | 0.0006 | 0.0004 | 0.0002 |
| Exxon AX03BE3 Part Size (in) | |||
| DI | DII | DIII | |
| 1 | 2.4375 | 2.4510 | 2.4520 |
| 2 | 2.4370 | 2.4515 | 2.4520 |
| 3 | 2.4370 | 2.4510 | 2.4515 |
| 4 | 2.4375 | 2.4515 | 2.4515 |
| 5 | 2.4375 | 2.4515 | 2.4515 |
| 6 | 2.4370 | 2.4510 | 2.4515 |
| 7 | 2.4360 | 2.4515 | 2.4515 |
| 8 | 2.4375 | 2.4520 | 2.4515 |
| 9 | 2.4370 | 2.4510 | 2.4515 |
| 10 | 2.4375 | 2.4520 | 2.4515 |
| High | 2.4375 | 2.4520 | 2.4520 |
| Average | 2.4372 | 2.4514 | 2.4516 |
| Low | 2.4360 | 2.4510 | 2.4515 |
| Range | 0.0015 | 0.0010 | 0.0005 |
| Standard Deviation | 0.0005 | 0.0004 | 0.0002 |
| Shrink Rate (in./in.) | |||
| Maximum | 0.0177 | 0.0117 | 0.0115 |
| Average | 0.0173 | 0.0115 | 0.0115 |
| Minimum | 0.0171 | 0.0113 | 0.0113 |
| Range | 0.0006 | 0.0004 | 0.0002 |
A Decoupled I process shows the largest shrink rate. This is because the wall freezes very quickly and does not allow for typical packing that compensates for shrinkage. Based on the material guidelines, the actual shrink rate is outside those limits by 0.0022 in./in. for the ABS. In comparison, the PP falls within the range near the center of the supplied range. When reviewing the changes in OAL, it has a range of 0.0015 inches.
Now let’s review how the material performed utilizing a Decoupled II strategy. For the ABS, the shrink rate is smaller but still outside the range provided by the material supplier by 0.0002 in/in. The same trend for shrink rate is observed in PP, but now is 0.0015 in./in. above the minimum shrinkage expected. With both materials, there is a reduction in OAL range of measurements from 0.0015 inches to 0.001 inches. The average length for the ABS was 0.005 inches longer, while the PP grew 0.0142 inches compared to its Decoupled I counterpart.
Onward to explore the results from the Decoupled III process. For both resins, the shrink rate is nearly identical, but the range in part OAL is reduced from 0.001 inches to 0.0005 inches compared to a Decoupled II process. If we review the part weight changes in PP and ABS, they are alike. This process has the tightest range for two reasons. First, both filling and packing phases are velocity controlled, allowing the machine to use the pressure needed to maintain the set velocity. This allows for two of three phases to compensate for changes in the material automatically by the machine. The second reason is the control of transfer is managed by the cavity pressure sensor not the screw position on the molding machine.
Part Weight
| Toyolac 100 Part Weight (grams) | |||
| DI | DII | DIII | |
| 1 | 1.3940 | 1.4675 | 1.4665 |
| 2 | 1.3932 | 1.4677 | 1.4667 |
| 3 | 1.3937 | 1.4679 | 1.4667 |
| 4 | 1.3923 | 1.4677 | 1.4667 |
| 5 | 1.3923 | 1.4675 | 1.4668 |
| 6 | 1.3939 | 1.4671 | 1.4666 |
| 7 | 1.3942 | 1.4676 | 1.4668 |
| 8 | 1.3942 | 1.4673 | 1.4669 |
| 9 | 1.3945 | 1.4673 | 1.4668 |
| 10 | 1.3921 | 1.4671 | 1.4669 |
| High | 1.3945 | 1.4679 | 1.4669 |
| Average | 1.3934 | 1.4675 | 1.4667 |
| Low | 1.3921 | 1.4671 | 1.4665 |
| Range | 0.0024 | 0.0008 | 0.0004 |
| Standard Deviation | 0.0009 | 0.0003 | 0.0001 |
| Exxon AC03BE3 Part Weight (grams) | |||
| DI | DII | DIII | |
| 1 | 1.1288 | 1.2560 | 1.2562 |
| 2 | 1.1280 | 1.2568 | 1.2571 |
| 3 | 1.1294 | 1.2568 | 1.2565 |
| 4 | 1.1289 | 1.2567 | 1.2571 |
| 5 | 1.1294 | 1.2574 | 1.2570 |
| 6 | 1.1297 | 1.2570 | 1.2568 |
| 7 | 1.1297 | 1.2572 | 1.2572 |
| 8 | 1.1301 | 1.2564 | 1.2571 |
| 9 | 1.1283 | 1.2565 | 1.2572 |
| 10 | 1.1293 | 1.2570 | 1.2573 |
| High | 1.1301 | 1.2574 | 1.2573 |
| Average | 1.1292 | 1.2568 | 1.2570 |
| Low | 1.1280 | 1.2560 | 1.2562 |
| Range | 0.0021 | 0.0014 | 0.0011 |
| Standard Deviation | 0.0007 | 0.0004 | 0.0004 |
For a Decoupled I process, the trend was essentially the difference between amorphous or semi-crystalline resins with weight varying 0.002 grams. One item we must take into account is that this part would likely never be molded using this process strategy as it’s not considered thin wall.
In a Decoupled II, part weight for both materials show an increase. For ABS, it’s 0.07 grams and 0.13 grams (Figure 5). These changes occur because of the pack/hold phase using a constant pressure to cram more polymer chains into the cavity to compensate for naturally occurring shrinkage that occurs during cooling.

Figure 5: Left ABS, Right PP weight change in pellets
The weight gain shifting to a Decoupled III process is 0.0008 grams for ABS and 0.0002 grams for PP.
Conclusion
In short, a Decoupled I process is best used for thin wall molding applications, Decoupled III is best for high tolerance molding, and Decoupled II is the industry standard for the majority of molded parts.
Trying to validate a part with the tight tolerances of ±0.002 inches and Cpk of 1.67 is going to be nearly impossible as the variation in OAL is using nearly the total tolerance band. The tried and true Decoupled II process will work for most projects as it produces parts with a range in OAL of 0.001 inches and a tolerance of ±0.002 inches, which would allow us to validate with a Cpk of 1.33, but not 1.67. The Decoupled III has a variation in OAL of 0.0005 inches, allowing a tighter tolerance of ±0.001 inches and a Cpk of 1.33.
Keep in mind, this is a very simple part with a single gate. As the project becomes more complex, choosing the correct process strategy is critical so the appropriate shrink rate can be cut into the mold cavity. This isn’t easy, but if it was, everyone would do it. Sit down as a team of part designers, mold designers, and molders and have healthy discussions so the project can be on time, under budget, and right the first time.
Nothing is impossible, we just need to do our due diligence as engineers.
Jeremy Williams has over 20 years of experience in the plastics industry serving the medical, automotive, furniture, and appliance industries. He previously worked as a Principal Engineer, taking projects from design concept to saleable products. Jeremy earned his Master Molder II certification in 2011, became an RJG Certified Trainer in 2012, and started at RJG in 2015. In addition to his extensive manufacturing background, he holds degrees in plastics and business. Currently Jeremy is a Consultant/Trainer with TZERO.