Becca Fantano, Marketing Communications Specialist, Performance Materials, Evonik Cyro01.14.19
In the world of disposable medical devices, component distributors rely heavily on off-the-shelf stock products to fulfill both large and small component orders. Whether meeting the needs of established OEMs, mid-sized device manufacturers, startups, or engineers developing and testing new designs, these distributors need to react quickly to changing industry demands while guaranteeing consistent quality across thousands of medical-grade parts.
In the case of Qosina, a global supplier of single-use medical device components, off-the-shelf components make up the majority of their product lines, which range from tubing, valves, and stopcocks to an array of highly specialized connectors. Being that Qosina was founded as a way for customers to eliminate the cost of tooling their own components and reap the benefits of immediate component delivery, the company must maintain a watchful eye on product availability.
The Need for Custom Molds and Specialized Products
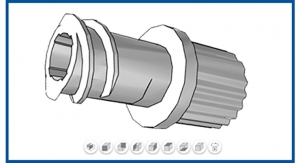
Qosina invariably comes upon situations where product procurement strategies need to be reassessed and adjusted. In 2016, such a case arose and Qosina found that it would no longer be able to keep up with customer demand for a specialized luer lock connector (Figure 1) due to increases in production times that were creating supply issues.
Qosina determined that making an investment in their own tooling and initiating their own molding program would give the company complete control over the connector in question. This would also eradicate any and all problems related to lead times and molding issues, as well as eliminate the potential for component cost increases that could be felt by Qosina’s customers.
The last piece of the equation was the material. To maintain production processes and end-user performance, the goal at this stage was to choose a component material that would mirror the previously used material and offer similar molding and usage properties.
“Delays in manufacturing were creating issues for us in terms of meeting customer demand in a timely fashion,” said Geri Trautman, product development manager at Qosina. “So, after we started working on our own tooling and our own molding source, we began talking with Evonik regarding the material that would best suit this new product. Evonik pointed us in a new direction, toward their CYROLITE G-20 HIFLO, a material that is easy to process and saves us considerable production costs over the long run.”
CYROLITE G-20 HIFLO Medical Acrylic
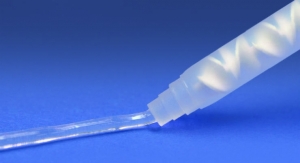
Evonik’s CYROLITE G-20 HIFLO acrylic-based copolymer compound is used primarily for the production of disposable medical devices (Figure 2). It has been in use in the medical market for over 30 years.
A BPA-free, impact-modified copolymer for the injection molding and extrusion of medical devices, CYROLITE G-20 HIFLO offers a number of benefits that made it an ideal fit for the Qosina luer lock connector. With a balance of physical properties and chemical resistance, CYROLITE offers excellent processability and flow characteristics for extremely thin-walled applications and complex multi-cavity molds. In addition, CYROLITE can be bonded to PVC tubing and sterilized by EtO gas and gamma irradiation.
“At this stage, we determined that CYROLITE was the optimal material for the part in question,” said Trautman. “We ended up with a better-performing material that we may not have even considered without Evonik’s input.”
Prototyping, Testing, and Production
Following prototyping and testing, Qosina was extremely satisfied to learn that this new luer lock connector, produced in-house through a partnership with an independent molding company, exceeded all performance specifications.
In addition, the use of CYROLITE G-20 HIFLO lowered the cost of production due to lower required mold temperatures, ease in processability, and faster cycle times. In the end, Qosina was able to pass these savings onto its customers, offering them a luer lock connector that delivered or exceeded all previous performance expectations at a lower cost.
“This efficiency in the production process, coupled with proven performance capabilities, made this a win-win across the board for us,” said Michael Gillis, supplier relations manager at Qosina. “We were able to make more parts in less time, enjoy less downtime on the molding machine, and also take advantage of energy cost savings due to increased productivity.”
In the case of CYROLITE G-20 HIFLO, many customers were already familiar with the material and product name, making the switch that much easier for Qosina and their customers.
With 100 percent of customers who had used the previous luer lock connector choosing to make the switch to the new product, Qosina has experienced an extremely successful transition to CYROLITE G-20 HIFLO. Up until this point, Qosina has also reported 100 percent customer satisfaction with the new luer lock connector, with no issues being reported in terms of usability, performance, or delivery times.
“When customers come to us for material recommendation, collaboration is important to ensure that all stakeholders share the application requirements and material knowledge. This is key to selecting the right material for the application and ensuring the long-term success of the medical device,” said Maurice Biagini, commercial director at Evonik. “In this case, CYROLITE G-20 HIFLO was the perfect choice for the application because it has the right balance of physical properties and excellent processability.”
A Successful Collaboration—A Promising Future
Though this was Qosina’s first foray into a product manufacturing arrangement of this nature, their partnership with Evonik has proven to be beneficial for both companies, as well as Qosina’s customers. Qosina is now positioned to continue to create their own components using their in-house tooling. In fact, Qosina has already begun a second project with Evonik under the same arrangement and is looking forward to the same results.
“Our partnership with Evonik is allowing us to better control costs, production, and the quality of our end product,” concluded Trautman. “Based on product demand, we will continue to look for areas where this approach makes sense for us. We look forward to continuing our partnership with Evonik for many years to come.”
“This project was an excellent example of collaboration between Evonik and Qosina. In fact, it was seamless every step of the way, which is sometimes a challenge in our industry,” added Biagini. “Both companies collaborated creatively and identified the best solution to meet the market needs. We are extremely excited about continuing the partnership that we have started with Qosina.”
In the case of Qosina, a global supplier of single-use medical device components, off-the-shelf components make up the majority of their product lines, which range from tubing, valves, and stopcocks to an array of highly specialized connectors. Being that Qosina was founded as a way for customers to eliminate the cost of tooling their own components and reap the benefits of immediate component delivery, the company must maintain a watchful eye on product availability.
The Need for Custom Molds and Specialized Products
Qosina invariably comes upon situations where product procurement strategies need to be reassessed and adjusted. In 2016, such a case arose and Qosina found that it would no longer be able to keep up with customer demand for a specialized luer lock connector (Figure 1) due to increases in production times that were creating supply issues.
Qosina determined that making an investment in their own tooling and initiating their own molding program would give the company complete control over the connector in question. This would also eradicate any and all problems related to lead times and molding issues, as well as eliminate the potential for component cost increases that could be felt by Qosina’s customers.
The last piece of the equation was the material. To maintain production processes and end-user performance, the goal at this stage was to choose a component material that would mirror the previously used material and offer similar molding and usage properties.
“Delays in manufacturing were creating issues for us in terms of meeting customer demand in a timely fashion,” said Geri Trautman, product development manager at Qosina. “So, after we started working on our own tooling and our own molding source, we began talking with Evonik regarding the material that would best suit this new product. Evonik pointed us in a new direction, toward their CYROLITE G-20 HIFLO, a material that is easy to process and saves us considerable production costs over the long run.”
CYROLITE G-20 HIFLO Medical Acrylic
Evonik’s CYROLITE G-20 HIFLO acrylic-based copolymer compound is used primarily for the production of disposable medical devices (Figure 2). It has been in use in the medical market for over 30 years.
A BPA-free, impact-modified copolymer for the injection molding and extrusion of medical devices, CYROLITE G-20 HIFLO offers a number of benefits that made it an ideal fit for the Qosina luer lock connector. With a balance of physical properties and chemical resistance, CYROLITE offers excellent processability and flow characteristics for extremely thin-walled applications and complex multi-cavity molds. In addition, CYROLITE can be bonded to PVC tubing and sterilized by EtO gas and gamma irradiation.
“At this stage, we determined that CYROLITE was the optimal material for the part in question,” said Trautman. “We ended up with a better-performing material that we may not have even considered without Evonik’s input.”
Prototyping, Testing, and Production
Following prototyping and testing, Qosina was extremely satisfied to learn that this new luer lock connector, produced in-house through a partnership with an independent molding company, exceeded all performance specifications.
In addition, the use of CYROLITE G-20 HIFLO lowered the cost of production due to lower required mold temperatures, ease in processability, and faster cycle times. In the end, Qosina was able to pass these savings onto its customers, offering them a luer lock connector that delivered or exceeded all previous performance expectations at a lower cost.
“This efficiency in the production process, coupled with proven performance capabilities, made this a win-win across the board for us,” said Michael Gillis, supplier relations manager at Qosina. “We were able to make more parts in less time, enjoy less downtime on the molding machine, and also take advantage of energy cost savings due to increased productivity.”
In the case of CYROLITE G-20 HIFLO, many customers were already familiar with the material and product name, making the switch that much easier for Qosina and their customers.
With 100 percent of customers who had used the previous luer lock connector choosing to make the switch to the new product, Qosina has experienced an extremely successful transition to CYROLITE G-20 HIFLO. Up until this point, Qosina has also reported 100 percent customer satisfaction with the new luer lock connector, with no issues being reported in terms of usability, performance, or delivery times.
“When customers come to us for material recommendation, collaboration is important to ensure that all stakeholders share the application requirements and material knowledge. This is key to selecting the right material for the application and ensuring the long-term success of the medical device,” said Maurice Biagini, commercial director at Evonik. “In this case, CYROLITE G-20 HIFLO was the perfect choice for the application because it has the right balance of physical properties and excellent processability.”
A Successful Collaboration—A Promising Future
Though this was Qosina’s first foray into a product manufacturing arrangement of this nature, their partnership with Evonik has proven to be beneficial for both companies, as well as Qosina’s customers. Qosina is now positioned to continue to create their own components using their in-house tooling. In fact, Qosina has already begun a second project with Evonik under the same arrangement and is looking forward to the same results.
“Our partnership with Evonik is allowing us to better control costs, production, and the quality of our end product,” concluded Trautman. “Based on product demand, we will continue to look for areas where this approach makes sense for us. We look forward to continuing our partnership with Evonik for many years to come.”
“This project was an excellent example of collaboration between Evonik and Qosina. In fact, it was seamless every step of the way, which is sometimes a challenge in our industry,” added Biagini. “Both companies collaborated creatively and identified the best solution to meet the market needs. We are extremely excited about continuing the partnership that we have started with Qosina.”