Matt Kosakowski, Business Development Director, Acumentrics12.04.18
Telehealth represents one of the most exciting industry trends because it enables more cost-effective and consistent care for patients around the world. According to Mark Britnell, chairman of KPMG’s Global Health Practice, consistency and continuity are needed to transform healthcare.1 By starting quality care at the earliest opportunity and facilitating seamless data collection, telehealth is a critical need. This recognition, both by medical professionals and the insurance industry, is driving significant growth.
Successful telehealth care demands that the associated portable medical equipment be operational at all times regardless of location, environment, or other challenging circumstances. Developing solutions for such applications is a formidable challenge that demands mastery of many disciplines, from software integration to wireless communications to medical data collection to effective user interfaces. Without such mastery, the telehealth system is simply a box of components difficult to configure and thus, of limited utility in the field. That said, the jack-of-all-trades-and-master-of-none effect is a reality in any industry. Therefore, outside expertise in critical areas that are not core competencies can help.
Power system expertise is one example for which telehealth suppliers can seek support. With an ever-growing range of sensors and instruments, telehealth power demand is increasing dramatically. However, by definition, power access in such applications is often unavailable or uncertain. Complete power autonomy is a must-have. Outside expertise can help telehealth systems suppliers achieve this requirement in a cost-effective and efficient way.
As a starting point, it is important to note that complete power autonomy requires more than just batteries and chargers. It is akin to a military mission: The ability to operate anywhere, at any time, and in any conditions is paramount.
Complete Power Autonomy Requirements
All of these requirements are relevant to current and future telehealth applications. For example:
Power Modules for Telehealth Cases and Backpacks
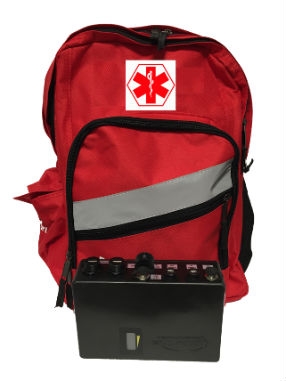
Acumentrics' Pack-Power small form factor UPS is ideal for backpack-based portable first responder systems. Image courtesy of Acumentrics.
Backpacks and cases equipped with computers, medical devices, LTE communications, and/or SATCOM are being used to provide care in remote locations, often in developing countries or when a patient has limited mobility and means of travel. The backpack is brought to the patient who may be in a clinic, home, or temporary shelter. Vital signs are obtained with the equipment in the case and diagnoses conducted via teleconference with specialists if necessary. Effective, in-depth medical care is delivered immediately.
In addition, the collected medical data is continuously transmitted for secure cloud storage and later access. As a result, an electronic medical history is created for the patient that can be reviewed and updated during subsequent care, fulfilling the need for consistency and continuity.
The pack may or may not have access to power in remote locations and must be completely self-supporting, often for extended periods of time. Even if external power is available, it may be “dirty” and/or unreliable, with voltage spikes, brownouts, blackouts, and other inconsistencies. Such power can cause damage and/or faulty indications. Flexibility and reliability, as previously described, are necessary to handle these realities. Furthermore, in addition to the power needed to operate the computer and communications equipment, most of the medical devices are operated and/or recharged via USB.
Robustness is mandatory. The remote locations in which these systems must operate may experience extreme heat or cold, humidity, dust, and/or direct exposure to the elements. The case may be thrown in the back of a vehicle or inadvertently dropped on the ground.
The devices within these cases and packs generate important medical data that typically needs to be consolidated and transmitted. Thus, in addition to enabling USB devices to operate, complete power autonomy also requires simultaneous data management capability. The power system must also function as a data bridge.

The Acumentrics Carry-On UPS Power Case can be operated untethered for long periods of time or can be charged while simultaneously providing USB, DC, and AC power. Image courtesy of Acumentrics.
In some cases, a telehealth system developer wants a higher level of integration. In these situations, purchasing a case with an embedded power system allows the supplier to add their desired assortment of devices, upload software, and ship to customers. As a starting point, the outside power expert conducts a thorough assessment in concert with the developer. Watts, amps, run time, charge time, battery chemistry, the operational environment, weight, size, and other factors are all considered in the system design. This approach saves the supplier valuable development time that would otherwise be spent selecting a case and implementing power capability.
As a result, the developer (and by extension, the end customer) obtains a power solution that is optimized in terms of performance and size. Supply chain costs are reduced because individual power components and cases do not need to be stocked.
Medical Cart Power
Medical carts are standard fixtures in many hospitals and clinics. These carts are used to collect vital signs, capture clinician/doctor observations, dispense medications, and allow remote telemedicine. In some cases, these carts can be plugged in when needed, but in many situations, the ability to operate untethered is valuable and necessary. In fact, the vast majority of telemedicine carts are already equipped with rechargeable battery power.
Anyone with outpatient or inpatient experience can appreciate the hectic, demanding schedules in a medical facility. Nurses and other clinicians must maintain the ability to operate an ever-growing range of equipment to a high standard while quickly moving from patient to patient. All the while, these professionals must do so with a positive attitude toward those receiving care, regardless of the time of day or length of shift. They rightly expect their tools to perform with minimal interruption.
A medical cart with a discharged battery is at best an inconvenience and at worst a liability. In the always-active hospital environment, it is unfair to ask those rendering critical care to also closely monitor their tools. There are simply too many other tasks at hand. A cart power system with long runtime and fast charging can help. With long runtimes, fewer systems are plugged into the wall and/or fewer batteries plugged into chargers. Carts are more likely to be available when needed. With faster charging, in the inevitable discharged situation, a couple of minutes will get the cart up and running so the job can get done. Flexibility, reliability, and robustness are all necessary.
Conclusion
Medical professionals, whether operating in a remote clinic, hospital, or in the field, deserve the best, easiest to use telehealth equipment. Regardless of sophistication, technology that is unreliable and/or difficult to use is a liability, not a benefit. Power expertise is just one area in which outside expertise is available to help telehealth developers provide optimal systems with rapid time to market.
Reference
1 Britnell, Mark, Transforming Health Care Takes Continuity and Consistency, Harvard Business Review, Dec. 28, 2015.
Successful telehealth care demands that the associated portable medical equipment be operational at all times regardless of location, environment, or other challenging circumstances. Developing solutions for such applications is a formidable challenge that demands mastery of many disciplines, from software integration to wireless communications to medical data collection to effective user interfaces. Without such mastery, the telehealth system is simply a box of components difficult to configure and thus, of limited utility in the field. That said, the jack-of-all-trades-and-master-of-none effect is a reality in any industry. Therefore, outside expertise in critical areas that are not core competencies can help.
Power system expertise is one example for which telehealth suppliers can seek support. With an ever-growing range of sensors and instruments, telehealth power demand is increasing dramatically. However, by definition, power access in such applications is often unavailable or uncertain. Complete power autonomy is a must-have. Outside expertise can help telehealth systems suppliers achieve this requirement in a cost-effective and efficient way.
As a starting point, it is important to note that complete power autonomy requires more than just batteries and chargers. It is akin to a military mission: The ability to operate anywhere, at any time, and in any conditions is paramount.
Complete Power Autonomy Requirements
-
Flexibility
- Ability to operate in the absence of external power
- Ability to recharge and power devices simultaneously
- Compatibility with local power infrastructure via worldwide AC input/output and DC input/output
-
Reliability
- Ability to withstand unexpected outages and provide clean, conditioned, uninterrupted power
-
Robustness
- Ability to operate in challenging environmental conditions
-
Data Management
- Ability to transmit information to/from medical devices
All of these requirements are relevant to current and future telehealth applications. For example:
Power Modules for Telehealth Cases and Backpacks

Acumentrics' Pack-Power small form factor UPS is ideal for backpack-based portable first responder systems. Image courtesy of Acumentrics.
In addition, the collected medical data is continuously transmitted for secure cloud storage and later access. As a result, an electronic medical history is created for the patient that can be reviewed and updated during subsequent care, fulfilling the need for consistency and continuity.
The pack may or may not have access to power in remote locations and must be completely self-supporting, often for extended periods of time. Even if external power is available, it may be “dirty” and/or unreliable, with voltage spikes, brownouts, blackouts, and other inconsistencies. Such power can cause damage and/or faulty indications. Flexibility and reliability, as previously described, are necessary to handle these realities. Furthermore, in addition to the power needed to operate the computer and communications equipment, most of the medical devices are operated and/or recharged via USB.
Robustness is mandatory. The remote locations in which these systems must operate may experience extreme heat or cold, humidity, dust, and/or direct exposure to the elements. The case may be thrown in the back of a vehicle or inadvertently dropped on the ground.
The devices within these cases and packs generate important medical data that typically needs to be consolidated and transmitted. Thus, in addition to enabling USB devices to operate, complete power autonomy also requires simultaneous data management capability. The power system must also function as a data bridge.

The Acumentrics Carry-On UPS Power Case can be operated untethered for long periods of time or can be charged while simultaneously providing USB, DC, and AC power. Image courtesy of Acumentrics.
As a result, the developer (and by extension, the end customer) obtains a power solution that is optimized in terms of performance and size. Supply chain costs are reduced because individual power components and cases do not need to be stocked.
Medical Cart Power
Medical carts are standard fixtures in many hospitals and clinics. These carts are used to collect vital signs, capture clinician/doctor observations, dispense medications, and allow remote telemedicine. In some cases, these carts can be plugged in when needed, but in many situations, the ability to operate untethered is valuable and necessary. In fact, the vast majority of telemedicine carts are already equipped with rechargeable battery power.
Anyone with outpatient or inpatient experience can appreciate the hectic, demanding schedules in a medical facility. Nurses and other clinicians must maintain the ability to operate an ever-growing range of equipment to a high standard while quickly moving from patient to patient. All the while, these professionals must do so with a positive attitude toward those receiving care, regardless of the time of day or length of shift. They rightly expect their tools to perform with minimal interruption.
A medical cart with a discharged battery is at best an inconvenience and at worst a liability. In the always-active hospital environment, it is unfair to ask those rendering critical care to also closely monitor their tools. There are simply too many other tasks at hand. A cart power system with long runtime and fast charging can help. With long runtimes, fewer systems are plugged into the wall and/or fewer batteries plugged into chargers. Carts are more likely to be available when needed. With faster charging, in the inevitable discharged situation, a couple of minutes will get the cart up and running so the job can get done. Flexibility, reliability, and robustness are all necessary.
Conclusion
Medical professionals, whether operating in a remote clinic, hospital, or in the field, deserve the best, easiest to use telehealth equipment. Regardless of sophistication, technology that is unreliable and/or difficult to use is a liability, not a benefit. Power expertise is just one area in which outside expertise is available to help telehealth developers provide optimal systems with rapid time to market.
Reference
1 Britnell, Mark, Transforming Health Care Takes Continuity and Consistency, Harvard Business Review, Dec. 28, 2015.