Dan Sanchez, R&D Engineering Manager, Principal Engineer, Trelleborg Sealing Solutions08.10.17
Its biocompatibility, flexibility, and durability have made extruded silicone a top choice for tubing in many medical devices. Of course, designing devices that will be attached to or lodged within the body is not the easiest of tasks, even with the positive qualities the material offers.
Today, silicone tubes are being enhanced with a variety of reinforcements and coatings that help manufacturers meet the market’s escalating demand for smaller, more durable devices. In this article, we’ll explore a number of such enhancements, with an emphasis on kink resistant tubing.
Spiral Design Adds Kink Resistance
Silicone is a soft material, and extrudable silicones are in the Shore-A durometer scale. This makes them prone to kinking, especially in small dimensions. As designs call for smaller device footprints and, as a result, tubes become smaller, tube diameters reach the point where, without reinforcement, they easily bend or collapse, halting the flow of fluid.
The solution is to embed monofilaments, either in a spiral or braided design, within the wall of the tube to add radial strength, making extruded silicone tubes highly resistant to kinking and compression. Today, small silicone tubes (1/8 inch to 3/16 inch) are available with a variety of reinforcements.
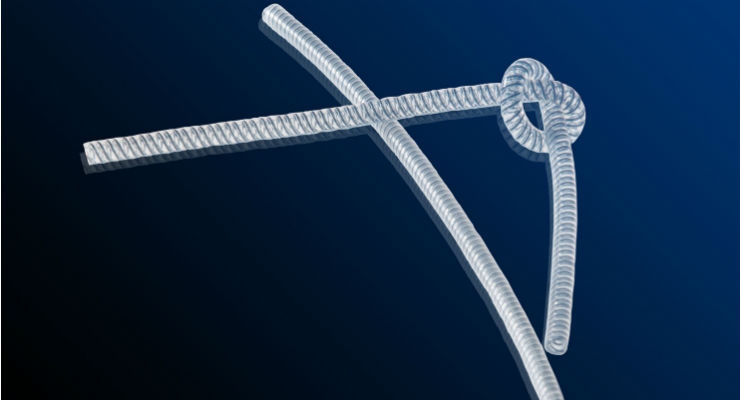
Monofilaments in a tight spiral design are ideal for adding kink resistance to small diameter tubes. The reinforcement adds enough radial strength so that tubing can be bent almost in half without affecting fluid flow. Because the monofilaments are embedded in the tube wall, they do not affect the inner diameter of the tube. This configuration is ideal for creating a small, highly flexible tube that will not kink or collapse, even when required to conform around anatomical features (Figure 1).
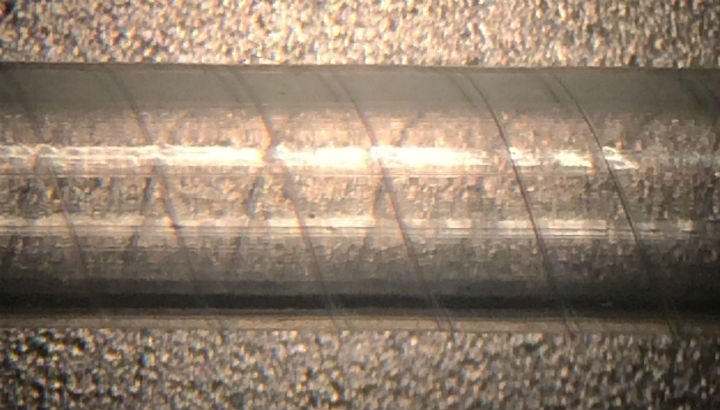
Figure 1: Kink-resistant tubing provides a pathway for the introduction or elimination of fluids from the body.
However, plastic reinforcements are sensitive to heat, so they cannot be used in devices that will be subjected to high temperatures, such as seen in autoclave sterilization. The silicone itself is highly heat resistant, but the autoclave’s high temperature would degrade the mechanical performance of the plastic embedded in the wall. In addition to plastic, spiral reinforcements can be manufactured from nylon and stainless steel, both of which are more heat resistant than plastic.
Kink resistant tubes can be used in a variety of medical devices, including catheters, pacemakers, and penile implants.
Braids Prevent Burst Tubes
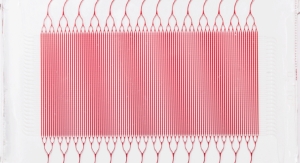
Some medical applications require tubes that are slightly less flexible than those that can be produced by spiral reinforcement but are stronger. Rather than kink resistance, the aim is to produce a tube that won’t expand, burst, or collapse under pressure. The solution is a braided monofilament, which can be constructed from a variety of plastics and metals, including polyethylene, nylon, and stainless steel (Figure 2).

Figure 2: Braided monofilament can be constructed from various materials for effective use in medical devices.
The flexibility of a braided monofilament depends on the number of crossed pieces in a given area. This is typically measured in per-inch crosses, or PICs. The higher the PICs, the tighter the braid; the tighter the braid, the less flexible the tube, but the stronger it will be.
Burst resistant tubes are currently being used in a variety of medical applications that require liquid (e.g., a drug or a flushing agent) to be delivered under pressure.
Reinforcers Extend Durability
Reinforced tubing can also be designed to increase wear resistance. Materials such as polyester and polyethylene can be placed inside the silicone wall in a spiral or braid configuration for tubes to be used inside the body.
One application example would be excess tubing coiled up and placed behind a pacemaker in the chest cavity. This may rub against the pacemaker due to arm/shoulder movement and over time, can cause the outer layer of silicone to break down. A smooth, durable reinforcement placed inside the silicone makes the tube considerably more wear resistant, extending the length of time the device can function inside the body before surgical replacement is required.
Wear resistant tubes can be used in a variety of implants such as heart pumps and cochlear devices.
Designing with Mandrels in Mind
Unlike thermoplastics, which must be melted to an exact point before extrusion and then cooled quickly to set the shape, high consistency rubber/elastomer (HCR/E) silicones are a thermoset material that rely on a chemical crosslinking process. HCEs have considerable green strength, making them an ideal material for extrusion, but once crosslinked with heat, will not re-melt.
However, the reinforcement process adds a layer of complexity to tubing manufacturing. The tube wall must be built on a mandrel while the spiral or braid is being created. The mandrel is removed once the reinforcement is complete.
Removing the mandrel from the tube can be difficult for long lengths of tubing, limiting the length of certain types of reinforced tubing. When long lengths are specified, one solution is to deliver the tube with the mandrel still inside. The customer cuts the tube to length, then removes the mandrel.
The size of the inner diameter (ID) is also a factor—larger tube IDs require a larger mandrel. As the mandrel increases in size, the surface area’s contact with the silicone enlarges significantly, increasing friction and making the mandrel more difficult to remove. Thus, the use of reinforcement in silicone tubes is currently limited to under 1/8-inch in diameter.
Thicker walls can also have a negative impact on removing the mandrel because they may have a tighter grip on the mandrel, increasing the friction coefficient between the silicone wall and the mandrel.
Another design consideration is the temperature threshold of the reinforcement material. Metals such as nitinol and stainless steel can be exposed to high heat without harm but not all plastics can. It would be more difficult to manufacture a tube using a low-melting-point plastic—temperatures that would successfully cure the silicone could compromise the reinforcement.
Keeping an Eye on Loose Ends
Since silicone doesn’t easily bond to other materials, the reinforcements described above are encapsulated into the silicone walls but not chemically bonded to them.
This is not a concern for applications where both ends of the reinforced tube are attached to another part of the device. If the tube was to be unattached at one end, however, it might need to be sealed through a secondary process to ensure that non-biocompatible (and possibly sharp) monofilaments don’t come into contact with the body or bodily fluids.
Depending upon the device being designed, the manufacturer may need to prove the biocompatibility of the reinforcement, even if it is unlikely to come into contact with the body. Some manufacturers specify materials such as stainless steel or nitinol because they have already proven to be biocompatible. Others choose to work with regulators to get new materials approved.
Considering Colors and Coatings
Silicone is naturally clear or translucent, but tubes can be pigmented in a variety of opaque or translucent colors. However, when specifying a reinforced tube, it’s wise to choose one that is clear, translucent, or has a translucent color. This ensures the reinforcement can be easily inspected to ascertain whether or not the correct number of coils in a spiral (or PICs in a braid) are present in the reinforcement.
A variety of silicone coatings are also available. Other than the heat necessary to cure the coating, there are no additional obstacles to coating a reinforced tube. Because uncoated silicone tubes tend to have a slightly tacky surface, friction-reducing surface coatings are often added to reduce friction within the device or within the body.
Conclusion
To meet market demand for smaller, more durable medical devices, silicone tubes are being enhanced with a variety of reinforcements and coatings. Silicone’s natural flexibility lends itself to tubing, with extrusions from 20 durometers to 80 durometers possible on the Shore-A scale. (20-A durometer is not recommended due to increased lot variability, gel presence, and difficulty in processing.)
A spiral reinforcement in a variety of coil configurations and materials can be embedded into the tube wall, adding kink resistance. Stronger reinforcements in a braid design can help increase wear and burst resistance, and coatings can be added to reinforced tubes to decrease friction.
Considerations for device designs with reinforced tubing include length (reinforcements are built around a mandrel that can be difficult to remove from long lengths), diameter (reinforced tubes must be under 1/8 inch ID), reinforcement material (plastic coils may not be able to withstand high temperatures), the need to seal any non-connected end to prevent harm from the sharp end of the monofilament, and biocompatibility of the reinforcement.
Today, silicone tubes are being enhanced with a variety of reinforcements and coatings that help manufacturers meet the market’s escalating demand for smaller, more durable devices. In this article, we’ll explore a number of such enhancements, with an emphasis on kink resistant tubing.
Spiral Design Adds Kink Resistance
Silicone is a soft material, and extrudable silicones are in the Shore-A durometer scale. This makes them prone to kinking, especially in small dimensions. As designs call for smaller device footprints and, as a result, tubes become smaller, tube diameters reach the point where, without reinforcement, they easily bend or collapse, halting the flow of fluid.
The solution is to embed monofilaments, either in a spiral or braided design, within the wall of the tube to add radial strength, making extruded silicone tubes highly resistant to kinking and compression. Today, small silicone tubes (1/8 inch to 3/16 inch) are available with a variety of reinforcements.
Monofilaments in a tight spiral design are ideal for adding kink resistance to small diameter tubes. The reinforcement adds enough radial strength so that tubing can be bent almost in half without affecting fluid flow. Because the monofilaments are embedded in the tube wall, they do not affect the inner diameter of the tube. This configuration is ideal for creating a small, highly flexible tube that will not kink or collapse, even when required to conform around anatomical features (Figure 1).

Figure 1: Kink-resistant tubing provides a pathway for the introduction or elimination of fluids from the body.
However, plastic reinforcements are sensitive to heat, so they cannot be used in devices that will be subjected to high temperatures, such as seen in autoclave sterilization. The silicone itself is highly heat resistant, but the autoclave’s high temperature would degrade the mechanical performance of the plastic embedded in the wall. In addition to plastic, spiral reinforcements can be manufactured from nylon and stainless steel, both of which are more heat resistant than plastic.
Kink resistant tubes can be used in a variety of medical devices, including catheters, pacemakers, and penile implants.
Braids Prevent Burst Tubes
Some medical applications require tubes that are slightly less flexible than those that can be produced by spiral reinforcement but are stronger. Rather than kink resistance, the aim is to produce a tube that won’t expand, burst, or collapse under pressure. The solution is a braided monofilament, which can be constructed from a variety of plastics and metals, including polyethylene, nylon, and stainless steel (Figure 2).

Figure 2: Braided monofilament can be constructed from various materials for effective use in medical devices.
The flexibility of a braided monofilament depends on the number of crossed pieces in a given area. This is typically measured in per-inch crosses, or PICs. The higher the PICs, the tighter the braid; the tighter the braid, the less flexible the tube, but the stronger it will be.
Burst resistant tubes are currently being used in a variety of medical applications that require liquid (e.g., a drug or a flushing agent) to be delivered under pressure.
Reinforcers Extend Durability
Reinforced tubing can also be designed to increase wear resistance. Materials such as polyester and polyethylene can be placed inside the silicone wall in a spiral or braid configuration for tubes to be used inside the body.
One application example would be excess tubing coiled up and placed behind a pacemaker in the chest cavity. This may rub against the pacemaker due to arm/shoulder movement and over time, can cause the outer layer of silicone to break down. A smooth, durable reinforcement placed inside the silicone makes the tube considerably more wear resistant, extending the length of time the device can function inside the body before surgical replacement is required.
Wear resistant tubes can be used in a variety of implants such as heart pumps and cochlear devices.
Designing with Mandrels in Mind
Unlike thermoplastics, which must be melted to an exact point before extrusion and then cooled quickly to set the shape, high consistency rubber/elastomer (HCR/E) silicones are a thermoset material that rely on a chemical crosslinking process. HCEs have considerable green strength, making them an ideal material for extrusion, but once crosslinked with heat, will not re-melt.
However, the reinforcement process adds a layer of complexity to tubing manufacturing. The tube wall must be built on a mandrel while the spiral or braid is being created. The mandrel is removed once the reinforcement is complete.
Removing the mandrel from the tube can be difficult for long lengths of tubing, limiting the length of certain types of reinforced tubing. When long lengths are specified, one solution is to deliver the tube with the mandrel still inside. The customer cuts the tube to length, then removes the mandrel.
The size of the inner diameter (ID) is also a factor—larger tube IDs require a larger mandrel. As the mandrel increases in size, the surface area’s contact with the silicone enlarges significantly, increasing friction and making the mandrel more difficult to remove. Thus, the use of reinforcement in silicone tubes is currently limited to under 1/8-inch in diameter.
Thicker walls can also have a negative impact on removing the mandrel because they may have a tighter grip on the mandrel, increasing the friction coefficient between the silicone wall and the mandrel.
Another design consideration is the temperature threshold of the reinforcement material. Metals such as nitinol and stainless steel can be exposed to high heat without harm but not all plastics can. It would be more difficult to manufacture a tube using a low-melting-point plastic—temperatures that would successfully cure the silicone could compromise the reinforcement.
Keeping an Eye on Loose Ends
Since silicone doesn’t easily bond to other materials, the reinforcements described above are encapsulated into the silicone walls but not chemically bonded to them.
This is not a concern for applications where both ends of the reinforced tube are attached to another part of the device. If the tube was to be unattached at one end, however, it might need to be sealed through a secondary process to ensure that non-biocompatible (and possibly sharp) monofilaments don’t come into contact with the body or bodily fluids.
Depending upon the device being designed, the manufacturer may need to prove the biocompatibility of the reinforcement, even if it is unlikely to come into contact with the body. Some manufacturers specify materials such as stainless steel or nitinol because they have already proven to be biocompatible. Others choose to work with regulators to get new materials approved.
Considering Colors and Coatings
Silicone is naturally clear or translucent, but tubes can be pigmented in a variety of opaque or translucent colors. However, when specifying a reinforced tube, it’s wise to choose one that is clear, translucent, or has a translucent color. This ensures the reinforcement can be easily inspected to ascertain whether or not the correct number of coils in a spiral (or PICs in a braid) are present in the reinforcement.
A variety of silicone coatings are also available. Other than the heat necessary to cure the coating, there are no additional obstacles to coating a reinforced tube. Because uncoated silicone tubes tend to have a slightly tacky surface, friction-reducing surface coatings are often added to reduce friction within the device or within the body.
Conclusion
To meet market demand for smaller, more durable medical devices, silicone tubes are being enhanced with a variety of reinforcements and coatings. Silicone’s natural flexibility lends itself to tubing, with extrusions from 20 durometers to 80 durometers possible on the Shore-A scale. (20-A durometer is not recommended due to increased lot variability, gel presence, and difficulty in processing.)
A spiral reinforcement in a variety of coil configurations and materials can be embedded into the tube wall, adding kink resistance. Stronger reinforcements in a braid design can help increase wear and burst resistance, and coatings can be added to reinforced tubes to decrease friction.
Considerations for device designs with reinforced tubing include length (reinforcements are built around a mandrel that can be difficult to remove from long lengths), diameter (reinforced tubes must be under 1/8 inch ID), reinforcement material (plastic coils may not be able to withstand high temperatures), the need to seal any non-connected end to prevent harm from the sharp end of the monofilament, and biocompatibility of the reinforcement.