Tony Freeman, Managing Director, Manning Advisors LLC06.09.17
Few supply chain executives begin their day worrying about the effect of health insurance reimbursement paradigms on medical device OEM product strategies. While such discussions can be as dry as the Mojave Desert, like the San Andreas fault lying under that patch of dry ground, this rarely considered topic may have a profound effect on the device industry. The rise of value-based payment will have more effect deciding winners and losers in the device industry and its supply chain than any other factor in the next three to five years.
As value-based reimbursement shift incentives to better medical outcomes, device OEMs are redesigning their product lines. Suppliers capable of assisting with a markedly new set of product requirements in the next few years stand to expand their business.
What is Value-Based Reimbursement?
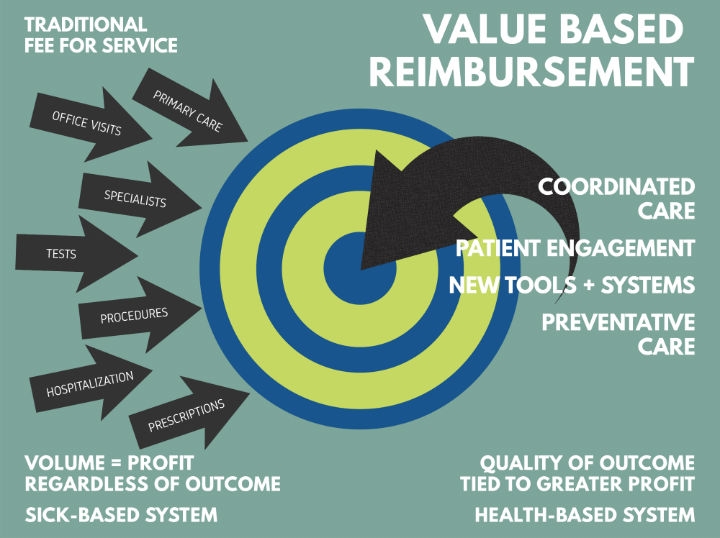
Value-based reimbursement (VBR), also called fee-for-value, is rapidly becoming the leading method for insurers to pay hospitals, doctors, and other healthcare providers. To understand VBR, it may be best to discuss the payment system most in the United States experienced for most of their lives—fee-for-service reimbursement (FFS). Under FFS, a doctor or hospital (i.e., provider) receives a payment every time they treat a patient. This seems fair until it is recognized that the provider gets paid whether the patient gets better or not. Under FFS, the incentive is to provide as many high-paying procedures as possible. There is less incentive to offer preventative medicine or recognition of providers who help patients avoid complications that may bring them back for additional costly, FFS-covered procedures. While FFS does a great job incentivizing the medical community to offer sophisticated care to as many people as possible, it does not provide motivation for keeping people healthy and out of the medical system.
VBR, on the other hand, harnesses economics to improve medical outcomes for patients and reduce healthcare costs. Providers usually receive a single payment covering all aspects of treatment. Those providers who consistently produce better outcomes (less hospital days, fewer complications) may keep more of the payment. Below-average providers have an economic incentive to improve or leave the field. VBR also creates incentives to prevent relapses and complications as the treatment costs will come out of that single payment. With the U.S. healthcare costs touching 18 percent of GDP, VBR provides a good balance of access and healthy, affordable outcomes.
How Pervasive is VBR?
VBR is a large slice of the reimbursement market and is growing rapidly. Between 25 and 30 percent of healthcare payments to U.S. providers in 2016 were made under VBR programs. By 2020, VBR will cover approximately 60 percent of the reimbursements. As the soon-to-be predominant means for paying hospitals, physicians, and other healthcare providers, its incentives for superior outcomes will change medical training, procedures, processes, and devices.
VBR and Medical Device Design/Sales
On the surface, it may appear the medical device OEMs would push back against VBR as it does not reward the high consumption of devices. Instead, what OEMs have learned is that there are opportunities for new, market-grabbing devices that offer both superior therapeutic results and competitive advantage over OEMs less agile in exploiting this new trend. Two cases demonstrate the point:
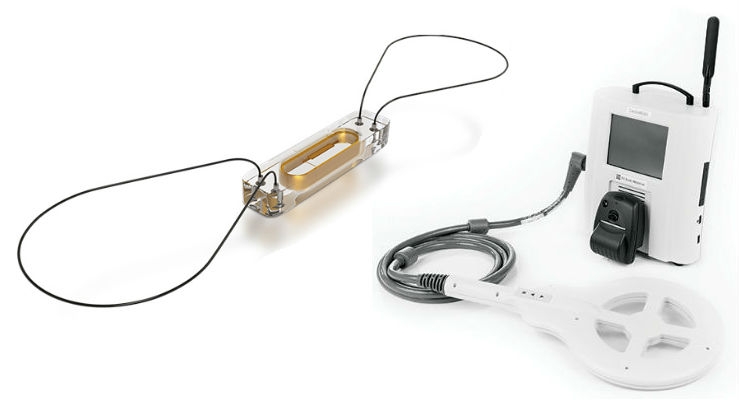
Case: St. Jude’s CaridoMEMs HF
For patients at risk of heart failure, a CardioMEMs wireless sensor is implanted in the pulmonary artery. The sensor uploads blood pressure readings including those that provide early warning of heart failure. In many cases, emergency room visits can be avoided by simply changing the patient’s medication. Core to St. Jude’s marketing message is that CardioMEMs saves an average of $10,640 in hospital costs per patient over three years.

Boston Scientific’s Watchman
Case: Boston Scientific’s Watchman
Watchman is a left atrial appendage closure (LAAC) device. Designed to prevent blood clots from reaching critical organs, it functions like a fuel filter in a car. As valuable as the product may be therapeutically, Boston Scientific’s marketing highlights its economic value as well. Part of the company’s pitch is that 40 percent of patients taking blood thinners miss dosages, putting them at risk for clots, which can lead to strokes. As stroke is one of the most disabling and expensive conditions to treat, presenting Watchman as an intelligent precautionary device demonstrates the company’s awareness of making products attractive in a VBR world.
VBR and the Supply Chain
Medical device OEMs will continue to adapt to the new method in which hospitals and physicians are being paid. Supply chain companies should watch for:
Changing the way nations pay for healthcare will have a profound effect on device OEMs and their suppliers. Suppliers should see VBR as an opportunity to offer their unique expertise to OEM customers creating new product lines.
Tony Freeman is the managing director of Manning Advisors LLC, a merger and acquisitions advisory firm based in New York City. He can be reached at tfreeman@manningadvisors.com.
As value-based reimbursement shift incentives to better medical outcomes, device OEMs are redesigning their product lines. Suppliers capable of assisting with a markedly new set of product requirements in the next few years stand to expand their business.
What is Value-Based Reimbursement?
Value-based reimbursement (VBR), also called fee-for-value, is rapidly becoming the leading method for insurers to pay hospitals, doctors, and other healthcare providers. To understand VBR, it may be best to discuss the payment system most in the United States experienced for most of their lives—fee-for-service reimbursement (FFS). Under FFS, a doctor or hospital (i.e., provider) receives a payment every time they treat a patient. This seems fair until it is recognized that the provider gets paid whether the patient gets better or not. Under FFS, the incentive is to provide as many high-paying procedures as possible. There is less incentive to offer preventative medicine or recognition of providers who help patients avoid complications that may bring them back for additional costly, FFS-covered procedures. While FFS does a great job incentivizing the medical community to offer sophisticated care to as many people as possible, it does not provide motivation for keeping people healthy and out of the medical system.
VBR, on the other hand, harnesses economics to improve medical outcomes for patients and reduce healthcare costs. Providers usually receive a single payment covering all aspects of treatment. Those providers who consistently produce better outcomes (less hospital days, fewer complications) may keep more of the payment. Below-average providers have an economic incentive to improve or leave the field. VBR also creates incentives to prevent relapses and complications as the treatment costs will come out of that single payment. With the U.S. healthcare costs touching 18 percent of GDP, VBR provides a good balance of access and healthy, affordable outcomes.

How Pervasive is VBR?
VBR is a large slice of the reimbursement market and is growing rapidly. Between 25 and 30 percent of healthcare payments to U.S. providers in 2016 were made under VBR programs. By 2020, VBR will cover approximately 60 percent of the reimbursements. As the soon-to-be predominant means for paying hospitals, physicians, and other healthcare providers, its incentives for superior outcomes will change medical training, procedures, processes, and devices.
VBR and Medical Device Design/Sales
On the surface, it may appear the medical device OEMs would push back against VBR as it does not reward the high consumption of devices. Instead, what OEMs have learned is that there are opportunities for new, market-grabbing devices that offer both superior therapeutic results and competitive advantage over OEMs less agile in exploiting this new trend. Two cases demonstrate the point:
Case: St. Jude’s CaridoMEMs HF
For patients at risk of heart failure, a CardioMEMs wireless sensor is implanted in the pulmonary artery. The sensor uploads blood pressure readings including those that provide early warning of heart failure. In many cases, emergency room visits can be avoided by simply changing the patient’s medication. Core to St. Jude’s marketing message is that CardioMEMs saves an average of $10,640 in hospital costs per patient over three years.

Boston Scientific’s Watchman
Watchman is a left atrial appendage closure (LAAC) device. Designed to prevent blood clots from reaching critical organs, it functions like a fuel filter in a car. As valuable as the product may be therapeutically, Boston Scientific’s marketing highlights its economic value as well. Part of the company’s pitch is that 40 percent of patients taking blood thinners miss dosages, putting them at risk for clots, which can lead to strokes. As stroke is one of the most disabling and expensive conditions to treat, presenting Watchman as an intelligent precautionary device demonstrates the company’s awareness of making products attractive in a VBR world.
VBR and the Supply Chain
Medical device OEMs will continue to adapt to the new method in which hospitals and physicians are being paid. Supply chain companies should watch for:
- More redesigns by OEMs to demonstrate superior outcomes and lower total costs of care. This does not mean the price of devices will decline. Rather, through superior design, OEMs will attempt to displace other costly therapies. In past years, OEMs would introduce modestly improved devices to stimulate sales. VBR pressures device manufacturers to show noticeable improvements.
- Product lines increasingly becoming systems rather than just devices. To deliver superior outcomes, OEMs recognize the therapies surrounding the use of the device are essential to healing time and complications. Increasingly, they will bundle devices with other devices, new procedures, expanded training, and data collection to ensure their products are delivering demonstrably superior results.
- OEM outreach to select supply chain partners capable of providing device design services around specific disease states. Historically, device OEMs have been slow to get new products out the door. The rapidly changing rewards of VBR accelerate the need to get to products to market in order to hold or gain market share. Supply chain partners capable of bringing a VBR-friendly product to market more quickly than their customers will be welcomed, assuming they can demonstrate their product’s benefits in a VBR world.
- Data system integration such as the embedding of sensors and development of interfaces will increase in a VBR world. VBR relies on continual benchmarking for two reasons. The first is that many conditions are best dealt with as soon as the disease is identified, making a device’s ability to communicate with a care team essential. The second is to demonstrate the ongoing value of the device. For devices to be accepted for reimbursement, a VBR philosophy requires they be demonstrated both therapeutically and financially superior to other therapies. While today’s medical devices may be among the most technologically sophisticated products in the world, they often are among the least networked. VBR requires this deficiency be remedied.
Changing the way nations pay for healthcare will have a profound effect on device OEMs and their suppliers. Suppliers should see VBR as an opportunity to offer their unique expertise to OEM customers creating new product lines.
Tony Freeman is the managing director of Manning Advisors LLC, a merger and acquisitions advisory firm based in New York City. He can be reached at tfreeman@manningadvisors.com.