Infographics
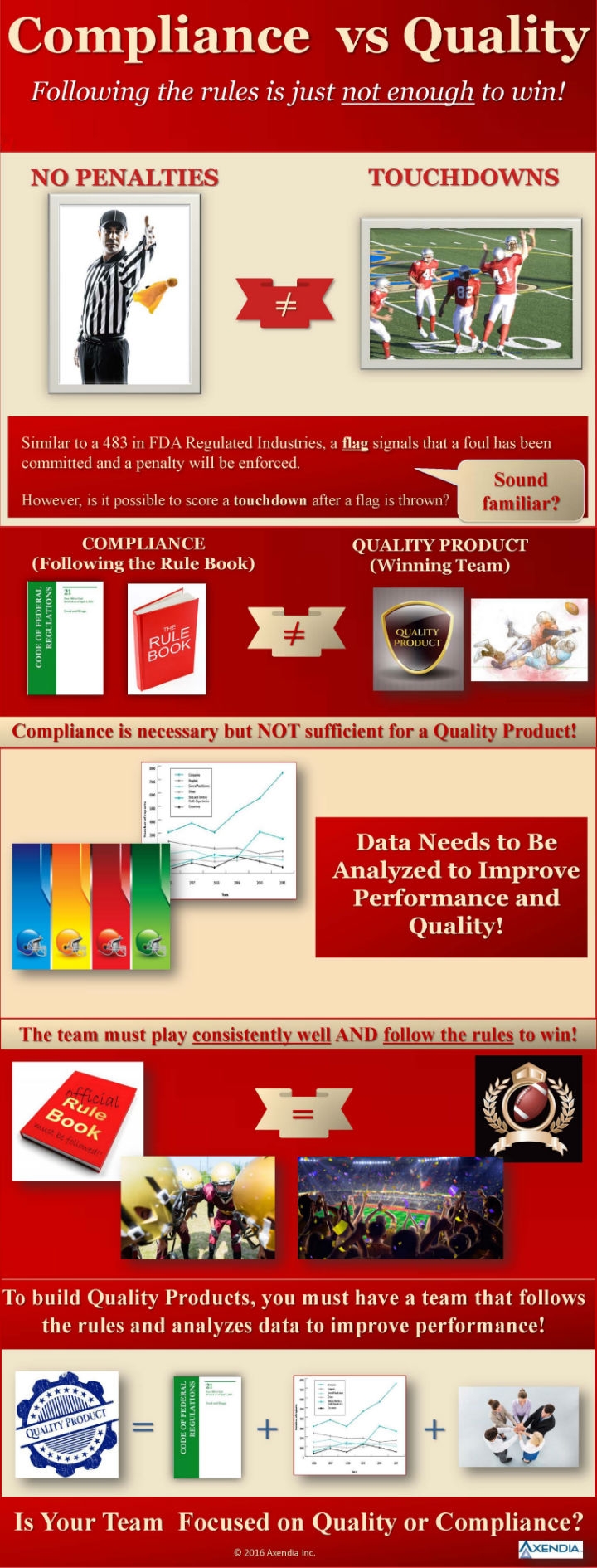
Compliance and Quality Part 1—Following the Rules Isn't Enough to Win
Compliance and Quality Part 1—Following the Rules Isn't Enough to Win
In the game of compliance versus quality, avoiding penalties doesn't ensure success.
By Axendia 04.11.16
For further insights on the topics and concepts illustrated in this infographic, check out "Can You Sell Poor Quality Devices in Compliance with Regulations? An examination of the often-misunderstood relationship between quality and compliance," by Daniel R. Matlis, the president of Axendia Inc.
Also see Infographic: Compliance and Quality Part 2—Food for Thought
Also see Infographic: Compliance and Quality Part 2—Food for Thought

Related Searches:
Related Infographics



























