Sam Brusco, Associate Editor01.30.18
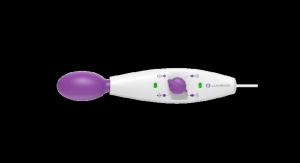
The AVACEN 100 (Advanced VAscular Circulation ENhancement) is an FDA-cleared, over-the-counter, non-invasive heat therapy device indicated for temporary relief of minor muscle and joint pain stiffness, joint pain associated with arthritis, muscle spasms, and minor strains and sprains, as well as muscular relaxation. Patients place their hand in the device for a 15-minute treatment session as a drug-free alternative.
As of mid-January, AVACEN 100 is also being trialed in India to relieve autistic behavior in children.
In case that seemed to come out of left field—it (gradually) did. AVACEN Medical, the maker of the device, has been receiving reports for years from parents who initially purchased the device for themselves that detailed relief of autistic behavior in their ASD (Autism Spectrum Disorder) children. The reported improvements ranged from rapidly disappearing speech impediments, to bolstered classroom participation and enthusiastic completion of homework, to school grades ballooning from Ds to As and Bs in a matter of months.
“Within the first two weeks of using the AVACEN 100 device for 30 minutes, twice a day, my 6-year-old son shocked us by reading a children’s book that he had never read before; from cover to cover, out loud, using his finger to point and perfectly pronounce each word,” Gavin Wiswell, a healthcare executive from San Marcos, Calif., recounted in a press release announcing the launch of AVACEN 100’s trial in India. “He typically would recite them back to us from memory, so this was a major breakthrough for our son!”
AVACEN’s breakthrough draws upon a theory that has existed since the 1980s—the “fever effect.” The phenomenon has its origins in a viral infection that swept through a therapeutic nursery for autistic children in New York’s Bellevue Psychiatric Hospital. The staff there reported that while feverish, the children were more social, alert, and talkative. But when the fever faded, so did the behavioral improvements. Researchers then scrambled to unravel the fever effect to develop therapeutic means to improve the lives of individuals affected by autism.
One study, carried out by Columbia University graduate student Rebecca Grzadzinski and Weill Cornell Medicine psychiatrist and autism expert Catherine Lord, Ph.D., described the characteristics of youth most likely to experience the fever effect. They examined data from 2,152 children with autism, and found 17 percent of them had experienced the fever effect—and they all had lower nonverbal cognitive skills, less language, and more repetitive behaviors than those children who had never experienced the effect. But what could be happening to those children when temperatures rise?
“The short answer is, we don’t know,” Grzadzinski admitted in a September 2017 Interactive Autism Network at Kennedy Krieger Institute article. But she offered a longer answer—fever may cause temporary cellular or metabolic changes affecting the central nervous system, which in turn may affect behavior. Possible theories explaining this include changes to the actions of cells in the hypothalamus, the release of glutamine or taurine, or “heat shock” proteins produced in response to fever to protect from cellular damage.
Since any prospective treatment or further clinical studies on the fever effect involve intentionally inflicting a child with a raised temperature, there are ethical concerns at play. Would parents go so far as to raise their ASD child’s susceptibility to infection in order to stimulate a fever and alleviate the symptoms of autism? If they did, would it be considered a form of abuse? Could a “controlled infection” feasibly become a treatment?
Thankfully, AVACEN 100’s prospective trial eliminates many of those concerns. With approximately 3 million treatments and zero reported adverse effects, the AVACEN 100 is the only known medical device able to safely and noninvasively warm the body by rapidly infusing heat into the circulatory system.
“During our launch, we will be deeply discounting AVACEN 100s ordered by families with autistic children and shipped to India. In return for the lower price, families must agree to complete a daily diary describing their child’s progress at home and in school during the 60-day money-back trial period,” AVACEN Medical CEO Thomas G. Muehlbauer stated in the press release. “The feedback from these surveys will accompany clinical trial results we plan to submit to the FDA to gain approval to treat autism behavior with the AVACEN 100 in the U.S.”
As of mid-January, AVACEN 100 is also being trialed in India to relieve autistic behavior in children.
In case that seemed to come out of left field—it (gradually) did. AVACEN Medical, the maker of the device, has been receiving reports for years from parents who initially purchased the device for themselves that detailed relief of autistic behavior in their ASD (Autism Spectrum Disorder) children. The reported improvements ranged from rapidly disappearing speech impediments, to bolstered classroom participation and enthusiastic completion of homework, to school grades ballooning from Ds to As and Bs in a matter of months.
“Within the first two weeks of using the AVACEN 100 device for 30 minutes, twice a day, my 6-year-old son shocked us by reading a children’s book that he had never read before; from cover to cover, out loud, using his finger to point and perfectly pronounce each word,” Gavin Wiswell, a healthcare executive from San Marcos, Calif., recounted in a press release announcing the launch of AVACEN 100’s trial in India. “He typically would recite them back to us from memory, so this was a major breakthrough for our son!”
AVACEN’s breakthrough draws upon a theory that has existed since the 1980s—the “fever effect.” The phenomenon has its origins in a viral infection that swept through a therapeutic nursery for autistic children in New York’s Bellevue Psychiatric Hospital. The staff there reported that while feverish, the children were more social, alert, and talkative. But when the fever faded, so did the behavioral improvements. Researchers then scrambled to unravel the fever effect to develop therapeutic means to improve the lives of individuals affected by autism.
One study, carried out by Columbia University graduate student Rebecca Grzadzinski and Weill Cornell Medicine psychiatrist and autism expert Catherine Lord, Ph.D., described the characteristics of youth most likely to experience the fever effect. They examined data from 2,152 children with autism, and found 17 percent of them had experienced the fever effect—and they all had lower nonverbal cognitive skills, less language, and more repetitive behaviors than those children who had never experienced the effect. But what could be happening to those children when temperatures rise?
“The short answer is, we don’t know,” Grzadzinski admitted in a September 2017 Interactive Autism Network at Kennedy Krieger Institute article. But she offered a longer answer—fever may cause temporary cellular or metabolic changes affecting the central nervous system, which in turn may affect behavior. Possible theories explaining this include changes to the actions of cells in the hypothalamus, the release of glutamine or taurine, or “heat shock” proteins produced in response to fever to protect from cellular damage.
Since any prospective treatment or further clinical studies on the fever effect involve intentionally inflicting a child with a raised temperature, there are ethical concerns at play. Would parents go so far as to raise their ASD child’s susceptibility to infection in order to stimulate a fever and alleviate the symptoms of autism? If they did, would it be considered a form of abuse? Could a “controlled infection” feasibly become a treatment?
Thankfully, AVACEN 100’s prospective trial eliminates many of those concerns. With approximately 3 million treatments and zero reported adverse effects, the AVACEN 100 is the only known medical device able to safely and noninvasively warm the body by rapidly infusing heat into the circulatory system.
“During our launch, we will be deeply discounting AVACEN 100s ordered by families with autistic children and shipped to India. In return for the lower price, families must agree to complete a daily diary describing their child’s progress at home and in school during the 60-day money-back trial period,” AVACEN Medical CEO Thomas G. Muehlbauer stated in the press release. “The feedback from these surveys will accompany clinical trial results we plan to submit to the FDA to gain approval to treat autism behavior with the AVACEN 100 in the U.S.”