Sam Brusco, Associate Editor02.03.23
Aspivix, a developer of gynecological technologies, has earned U.S. Food and Drug Administration (FDA) 510(k) clearance for its Carevix cervical stabilizer.
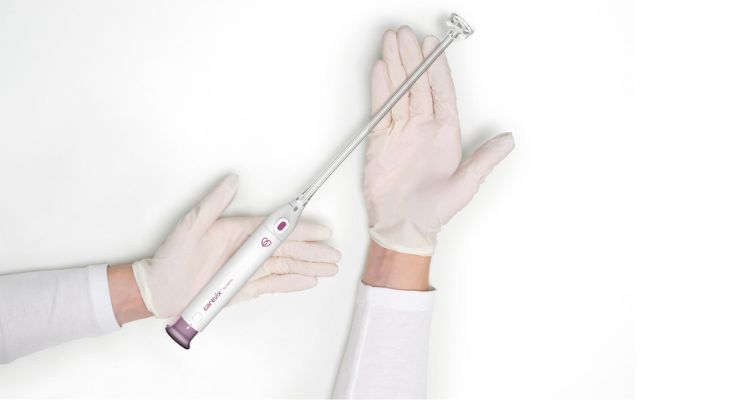
The Carevix atraumatic cervical stabilizer uses a gentle approach to reduce pain and bleeding for transcervical procedures like intrauterine device (IUD) insertions. The company’s ADVANCE study evaluated 100 women who underwent IUD insertion either with Carevix or a traditional cervical tenaculum: women reported “statistically significant results with up to 73% reduction of pain scores and 78% reduction of bleeding occurrences in favor of the Carevix device.”1

Carevix by Aspivix versus cervical tenaculum.
Mathieu Horras, CEO of Aspivix told the press: "With the 510(k) clearance of Carevix, a design-award winning device, we will provide our U.S. customers with an innovative and easy-to-use system that brings a gentler alternative to a century-old gynecological tool. Extensive research was incorporated into the development of Carevix so we know the unique and differentiating features it demonstrates with significant less pain and bleeding that has the potential to dramatically improve the IUD adoption and placement experience for millions of American women."
Reference
1 Dr. Yaron, Michal. "Safety and efficacy of an innovative atraumatic cervical stabilizer for IUD insertion: Results from a randomized, single blind controlled study." 30th World Congress on Controversies in Obstetrics, Gynecology, and Infertility (COGI), November 24-26, 2022, Amsterdam, The Netherlands. Oral Presentation.
The Carevix atraumatic cervical stabilizer uses a gentle approach to reduce pain and bleeding for transcervical procedures like intrauterine device (IUD) insertions. The company’s ADVANCE study evaluated 100 women who underwent IUD insertion either with Carevix or a traditional cervical tenaculum: women reported “statistically significant results with up to 73% reduction of pain scores and 78% reduction of bleeding occurrences in favor of the Carevix device.”1

Carevix by Aspivix versus cervical tenaculum.
Mathieu Horras, CEO of Aspivix told the press: "With the 510(k) clearance of Carevix, a design-award winning device, we will provide our U.S. customers with an innovative and easy-to-use system that brings a gentler alternative to a century-old gynecological tool. Extensive research was incorporated into the development of Carevix so we know the unique and differentiating features it demonstrates with significant less pain and bleeding that has the potential to dramatically improve the IUD adoption and placement experience for millions of American women."
Reference
1 Dr. Yaron, Michal. "Safety and efficacy of an innovative atraumatic cervical stabilizer for IUD insertion: Results from a randomized, single blind controlled study." 30th World Congress on Controversies in Obstetrics, Gynecology, and Infertility (COGI), November 24-26, 2022, Amsterdam, The Netherlands. Oral Presentation.