Charles Sternberg, Associate Editor03.03.22
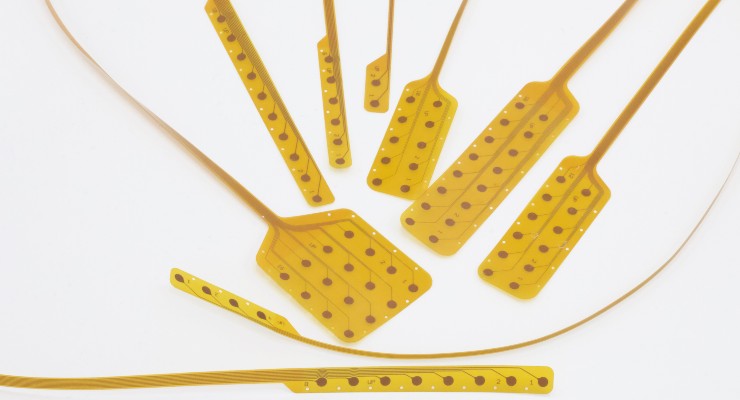
NeuroOne Medical Technologies Corporation, a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, has completed initial bench top, long-term stimulation testing on a novel design of its thin film electrode technology.
The tests measured the electrodes' ability to deliver the number of electrical stimulation pulses required to meet approximately five years of use using an active accelerated aging test model. The results demonstrated the potential to provide chronic stimulation at typical stimulation parameters currently used to treat patients suffering with chronic back pain due to multiple failed back surgeries.
Dave Rosa, CEO of NeuroOne commented, "Over the past few years we have been working to develop the technology for long term use given the large market opportunity for chronic stimulation technology. The results of both the long-term recording and stimulation testing demonstrate we are getting closer to achieving that goal.”
Rosa continued, “If we are successful in the ongoing development and subsequent submission to the FDA this would enable us to pursue expansion into therapeutic areas such as long-term stimulation for epilepsy, Parkinson's disease, chronic back pain due to failed back surgeries and other related neurological disorders. It may also present opportunities for licensing the technology to strategic partners for applications that are outside of our areas of focus.”
The company’s initial area of interest is expected to be spinal cord stimulation for chronic back pain given the size of the market and the competitive differentiator it can bring to this market with its unique thin film, low profile electrode platform.
The tests were conducted by recording the number of pulses of stimulation each pair of electrode contacts could deliver before identifying degradation. For a patient with chronic back pain receiving 16 hours of stimulation per day, the testing duration and intensity translates to over 5 years of operation or 4-5 years if operating 24 hours per day.
Camilo Diaz-Botia, PhD, director of Electrode Development for NeuroOne, said, "We conducted active accelerated longevity stimulation tests in saline solution, which is known to represent harsher conditions than saline solutions with added proteins. After several months of accelerated testing, we observed that most electrode contacts preserve the ability to deliver stimulation pulses at levels that would benefit most patients. We also observed that the electrode material remained electrochemically stable. Lastly, in agreement with our results on long-term recording, we again observed that the thin film insulation performed well during testing."
The company previously announced successful passive accelerated testing on electrodes that successfully demonstrated the ability to record electrical activity for a minimum of five years.
NeuroOne is also advancing a pipeline of therapeutic electrode technologies for brain tissue ablation, chronic stimulation use for DBS (deep brain stimulation) and spinal cord stimulation for chronic back pain. The company believes these therapeutic electrode technologies are targeted to addressable markets of over $10 billion.
The tests measured the electrodes' ability to deliver the number of electrical stimulation pulses required to meet approximately five years of use using an active accelerated aging test model. The results demonstrated the potential to provide chronic stimulation at typical stimulation parameters currently used to treat patients suffering with chronic back pain due to multiple failed back surgeries.
Dave Rosa, CEO of NeuroOne commented, "Over the past few years we have been working to develop the technology for long term use given the large market opportunity for chronic stimulation technology. The results of both the long-term recording and stimulation testing demonstrate we are getting closer to achieving that goal.”
Rosa continued, “If we are successful in the ongoing development and subsequent submission to the FDA this would enable us to pursue expansion into therapeutic areas such as long-term stimulation for epilepsy, Parkinson's disease, chronic back pain due to failed back surgeries and other related neurological disorders. It may also present opportunities for licensing the technology to strategic partners for applications that are outside of our areas of focus.”
The company’s initial area of interest is expected to be spinal cord stimulation for chronic back pain given the size of the market and the competitive differentiator it can bring to this market with its unique thin film, low profile electrode platform.
About the Tests
The company performed accelerated stimulation testing using stimulation levels above what is typically used today to treat patients suffering with chronic back pain due to multiple failed back surgeries.The tests were conducted by recording the number of pulses of stimulation each pair of electrode contacts could deliver before identifying degradation. For a patient with chronic back pain receiving 16 hours of stimulation per day, the testing duration and intensity translates to over 5 years of operation or 4-5 years if operating 24 hours per day.
Camilo Diaz-Botia, PhD, director of Electrode Development for NeuroOne, said, "We conducted active accelerated longevity stimulation tests in saline solution, which is known to represent harsher conditions than saline solutions with added proteins. After several months of accelerated testing, we observed that most electrode contacts preserve the ability to deliver stimulation pulses at levels that would benefit most patients. We also observed that the electrode material remained electrochemically stable. Lastly, in agreement with our results on long-term recording, we again observed that the thin film insulation performed well during testing."
The company previously announced successful passive accelerated testing on electrodes that successfully demonstrated the ability to record electrical activity for a minimum of five years.
NeuroOne is also advancing a pipeline of therapeutic electrode technologies for brain tissue ablation, chronic stimulation use for DBS (deep brain stimulation) and spinal cord stimulation for chronic back pain. The company believes these therapeutic electrode technologies are targeted to addressable markets of over $10 billion.