Michael Barbella, Managing Editor03.15.21
Rhythmlink International LLC has received U.S. Food and Drug Administration (FDA) clearance for a new portfolio of electrodes.
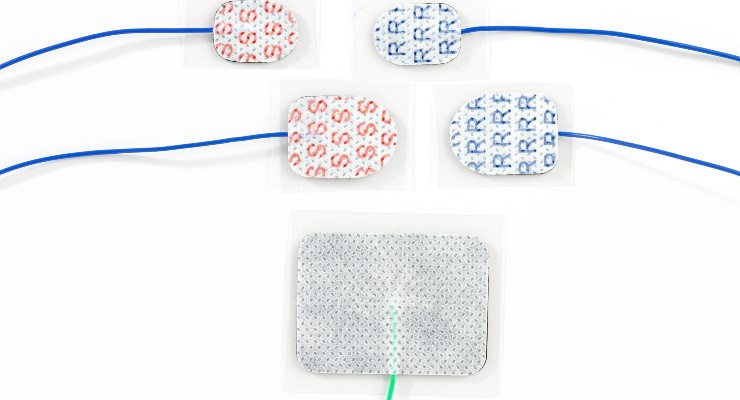
The agency recently greenlighted the company's Sticky Pad Surface Electrodes, a line of peel and stick-on electrodes for Intraoperative neurophysiological monitoring (IONM) and electroencephalography (EEG). The product line is now FDA cleared MR Conditional for 1.5 and 3 Tesla MRI environments.
“Medical professionals have historically used EEG Cups or Subdermal Needles for certain care situations, but this clearance unlocks additional benefits of the self-adhesive Sticky Pad Electrode,” said Leah Hanson, FASET, REEGT/EPT, CNIM [95-05] and vice president of Sales and Marketing for Rhythmlink.
Rhythmlink has been selling a line of disposable hydrogel Sticky Pad electrodes as part of their EEG and IONM product lines since 2007, but recently Rhythmlink sought and was granted FDA clearance on a modification of this line, allowing it to be used safely and effectively in imaging environments. Rhythmlink’s original Sticky Pad Electrode line set a new industry benchmark as the only Sticky Pad Electrode to be cleared for both recording and stimulating. This clearance allowed IONM professionals to use the three varieties of Sticky Pad electrodes, each specially designed for stimulating, grounding, or recording with greater confidence to achieve superior results. Each recording pad comes pre-gelled in formulated hydrogel to record high-quality signals while the stimulating pad is specifically designed to minimize stimulation risks or artifacts. The self-adhesive hydrogel design speeds up application and streamlines bedside workflow by eliminating extra steps to hold electrodes in place.
MR Conditional Sticky Pads will also be available in each of these three styles, in a small or large size to accommodate every user’s needs. Like many of Rhythmlink’s products, the MR Conditional Sticky Pad electrodes are single-patient use and fully disposable, reducing patient risk of cross contamination and creating an easy and more efficient workflow for medical staff. When a patient requires imaging, the electrodes can remain applied to the patient for the duration of their CT or MRI.
“In many cases both in critical care EEG or IONM surgeries, a CT or MRI is required. Until now Sticky Pads were not an option even though the electrode design is preferred,” Hanson explained. “In addition to being a lighter weight electrode with more surface area, the MR Conditional Sticky Pad Electrode can offer added patient comfort and protection to delicate skin areas by eliminating the need to remove and reapply the electrodes each time a CT or MRI is required. Because repeated removal and application can put the patient at a higher risk of skin breakdown and injury, it is precisely for these patients that it was crucial to make an electrode that can safely enter an imaging environment.”
A self-adhesive Sticky Pad Electrode also saves valuable time during application. Hanson said, “Quicker electrode application when combined with the ability to keep electrodes applied for CT or MRI imaging can provide advanced patient care during moments where time is of the essence.”
The agency recently greenlighted the company's Sticky Pad Surface Electrodes, a line of peel and stick-on electrodes for Intraoperative neurophysiological monitoring (IONM) and electroencephalography (EEG). The product line is now FDA cleared MR Conditional for 1.5 and 3 Tesla MRI environments.
“Medical professionals have historically used EEG Cups or Subdermal Needles for certain care situations, but this clearance unlocks additional benefits of the self-adhesive Sticky Pad Electrode,” said Leah Hanson, FASET, REEGT/EPT, CNIM [95-05] and vice president of Sales and Marketing for Rhythmlink.
Rhythmlink has been selling a line of disposable hydrogel Sticky Pad electrodes as part of their EEG and IONM product lines since 2007, but recently Rhythmlink sought and was granted FDA clearance on a modification of this line, allowing it to be used safely and effectively in imaging environments. Rhythmlink’s original Sticky Pad Electrode line set a new industry benchmark as the only Sticky Pad Electrode to be cleared for both recording and stimulating. This clearance allowed IONM professionals to use the three varieties of Sticky Pad electrodes, each specially designed for stimulating, grounding, or recording with greater confidence to achieve superior results. Each recording pad comes pre-gelled in formulated hydrogel to record high-quality signals while the stimulating pad is specifically designed to minimize stimulation risks or artifacts. The self-adhesive hydrogel design speeds up application and streamlines bedside workflow by eliminating extra steps to hold electrodes in place.
MR Conditional Sticky Pads will also be available in each of these three styles, in a small or large size to accommodate every user’s needs. Like many of Rhythmlink’s products, the MR Conditional Sticky Pad electrodes are single-patient use and fully disposable, reducing patient risk of cross contamination and creating an easy and more efficient workflow for medical staff. When a patient requires imaging, the electrodes can remain applied to the patient for the duration of their CT or MRI.
“In many cases both in critical care EEG or IONM surgeries, a CT or MRI is required. Until now Sticky Pads were not an option even though the electrode design is preferred,” Hanson explained. “In addition to being a lighter weight electrode with more surface area, the MR Conditional Sticky Pad Electrode can offer added patient comfort and protection to delicate skin areas by eliminating the need to remove and reapply the electrodes each time a CT or MRI is required. Because repeated removal and application can put the patient at a higher risk of skin breakdown and injury, it is precisely for these patients that it was crucial to make an electrode that can safely enter an imaging environment.”
A self-adhesive Sticky Pad Electrode also saves valuable time during application. Hanson said, “Quicker electrode application when combined with the ability to keep electrodes applied for CT or MRI imaging can provide advanced patient care during moments where time is of the essence.”