Michael Barbella, Managing Editor03.02.21
BrainCool AB (publ) has scored a significant milestone with the U.S. Food and Drug Administration's (FDA) decision to award Breakthrough Device Designation to the Cooral System (for oral mucositis prevention).
"This is a significant milestone for the Cooral System," said Martin Waleij CEO BrainCool AB (publ). "Not only does this 'Breakthrough' Designation safeguard a faster and smoother regulatory process as we enter the U.S. market, the new CMS reimbursement ruling is a vital mechanism for getting the device into clinical practice where it can actively benefit patients without further delay."
The FDA's Breakthrough Devices Program, implemented in 2018, replaces other fast track programs, and is intended to increase delivery of innovative medical devices that "provide for more effective treatment or diagnosis of life-threatening, or irreversibly debilitating diseases." With this designation, the FDA provides priority review and feedback throughout the final stages of market clearance through a de novo 510(k) process, and continued support as the device enters the market in the United States.
In addition to Breakthrough Device designation, the Cooral System will benefit significantly from recent Centers for Medicare & Medicaid Services (CMS) reimbursement changes that ensure coverage of medical devices designated as breakthrough by the FDA. The Medicare Coverage of Innovative Technology (MCIT) (CMS-3372-P) rule, which was finalized on Jan. 12, stipulates that national Medicare coverage for devices with "Breakthrough" Designation begins on the date of FDA market authorization and continues for four years.
Cooral's FDA Breakthrough Designation and CMS reimbursement mark major progress in OM and cancer treatment options.
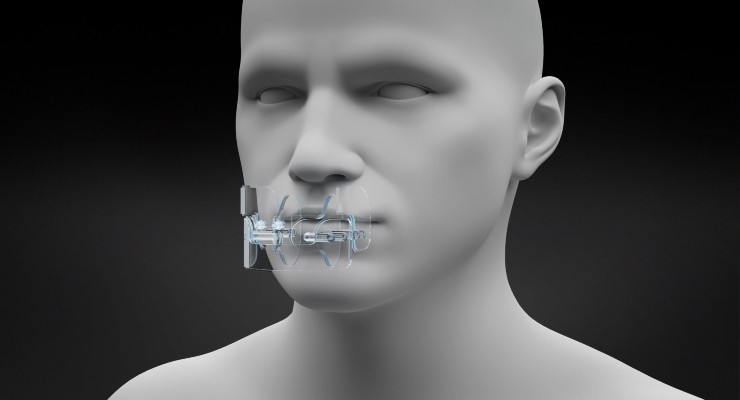
The Cooral System, CE marked in June 2020 as an invasive medical device in the EU, has shown statistically significant evidence of its safety and effectiveness in clinical trials. A study presented at a late breaking session during the 2020 European Society for Medical Oncology's Virtual Meeting in September, is expected to be published in a major scientific journal this spring.
As part of its application for FDA "Breakthrough Device" Designation, BrainCool AB (publ) presented already completed clinical results from the Nordic multi-center study assessing the safety and effectiveness of the Cooral System and showing significant evidence for prevention of oral mucositis (OM).
"We are confident that positive outcomes from recent clinical studies showing the Cooral System's ability to prevent oral mucositis were a major factor in winning this FDA Designation," added Waleij. "The double benefit of this Breakthrough Designation and CMS reimbursement decision represent a significant advance in cancer treatment options and the relief of patient suffering."
"Intra-oral cooling to prevent or mitigate oral mucositis represents an important, safe, and effective technology for selected cohorts of oncology patients," said Douglas E. Peterson, D.M.D., Ph.D., a consultant to BrainCool. "Based on my several decades of research and clinical experience relative to oral mucositis caused by cancer therapies, reduction of the severe pain and ulceration associated with the condition is key, especially for patients on aggressive treatment schedules for whom delays due to oral mucositis may be life-threatening."
"Oncologists all over the world are in need of a better cooling method to prevent Oral Mucositis and thus improving the quality of life for the patients," said Roger Henriksson, M.D., Ph.D., senior professor, University in Umeå. "Cooling is a well-known method to prevent this chemo-induced side effect, but it is only limitedly applied in clinics because using ice water is painful for the patient and poorly tolerated during the entire treatment time. Risk of infection can also be a deterrent. The Cooral System is an important step forward and much-needed technology."
OM is a highly significant and sometimes dose-limiting condition that has been reported as the single most-debilitating complication of cancer therapy. OM can be present in combination with a variety of debilitating symptoms that may compromise the ability of patients to maintain oral hygiene practices. For example, intractable oral pain, which may lead to an increased need for analgesics and, on occasions, opioids that are administered intravenously. OM is further associated with undernourishment, weight loss, the use of feeding tubes or total parenteral nutrition, and impaired quality of life, and it can represent a portal of entry for systemic infections that can lead to sepsis and death. Taken together, these symptoms, along with their related sequelae, can result in hospitalization and may incur increased costs for healthcare systems.
Based in Lund, Sweden, Europe, BrainCool AB (publ) (Spotlight markets BRAIN) is a publicly traded medical device company focused on next-generation temperature management systems. The company has two business units, braincooling, with the two CE marked products BrainCool System and RhinoChill, and in oncology with the Cooral System. BrainCool Inc., the U.S. subsidiary of BrainCool AB, is based in Annapolis, Md. The IQool System, which received FDA 510(k) clearance in 2018, was the first BrainCool product to be marketed in the United States.
"This is a significant milestone for the Cooral System," said Martin Waleij CEO BrainCool AB (publ). "Not only does this 'Breakthrough' Designation safeguard a faster and smoother regulatory process as we enter the U.S. market, the new CMS reimbursement ruling is a vital mechanism for getting the device into clinical practice where it can actively benefit patients without further delay."
The FDA's Breakthrough Devices Program, implemented in 2018, replaces other fast track programs, and is intended to increase delivery of innovative medical devices that "provide for more effective treatment or diagnosis of life-threatening, or irreversibly debilitating diseases." With this designation, the FDA provides priority review and feedback throughout the final stages of market clearance through a de novo 510(k) process, and continued support as the device enters the market in the United States.
In addition to Breakthrough Device designation, the Cooral System will benefit significantly from recent Centers for Medicare & Medicaid Services (CMS) reimbursement changes that ensure coverage of medical devices designated as breakthrough by the FDA. The Medicare Coverage of Innovative Technology (MCIT) (CMS-3372-P) rule, which was finalized on Jan. 12, stipulates that national Medicare coverage for devices with "Breakthrough" Designation begins on the date of FDA market authorization and continues for four years.
Cooral's FDA Breakthrough Designation and CMS reimbursement mark major progress in OM and cancer treatment options.
The Cooral System, CE marked in June 2020 as an invasive medical device in the EU, has shown statistically significant evidence of its safety and effectiveness in clinical trials. A study presented at a late breaking session during the 2020 European Society for Medical Oncology's Virtual Meeting in September, is expected to be published in a major scientific journal this spring.
As part of its application for FDA "Breakthrough Device" Designation, BrainCool AB (publ) presented already completed clinical results from the Nordic multi-center study assessing the safety and effectiveness of the Cooral System and showing significant evidence for prevention of oral mucositis (OM).
"We are confident that positive outcomes from recent clinical studies showing the Cooral System's ability to prevent oral mucositis were a major factor in winning this FDA Designation," added Waleij. "The double benefit of this Breakthrough Designation and CMS reimbursement decision represent a significant advance in cancer treatment options and the relief of patient suffering."
"Intra-oral cooling to prevent or mitigate oral mucositis represents an important, safe, and effective technology for selected cohorts of oncology patients," said Douglas E. Peterson, D.M.D., Ph.D., a consultant to BrainCool. "Based on my several decades of research and clinical experience relative to oral mucositis caused by cancer therapies, reduction of the severe pain and ulceration associated with the condition is key, especially for patients on aggressive treatment schedules for whom delays due to oral mucositis may be life-threatening."
"Oncologists all over the world are in need of a better cooling method to prevent Oral Mucositis and thus improving the quality of life for the patients," said Roger Henriksson, M.D., Ph.D., senior professor, University in Umeå. "Cooling is a well-known method to prevent this chemo-induced side effect, but it is only limitedly applied in clinics because using ice water is painful for the patient and poorly tolerated during the entire treatment time. Risk of infection can also be a deterrent. The Cooral System is an important step forward and much-needed technology."
OM is a highly significant and sometimes dose-limiting condition that has been reported as the single most-debilitating complication of cancer therapy. OM can be present in combination with a variety of debilitating symptoms that may compromise the ability of patients to maintain oral hygiene practices. For example, intractable oral pain, which may lead to an increased need for analgesics and, on occasions, opioids that are administered intravenously. OM is further associated with undernourishment, weight loss, the use of feeding tubes or total parenteral nutrition, and impaired quality of life, and it can represent a portal of entry for systemic infections that can lead to sepsis and death. Taken together, these symptoms, along with their related sequelae, can result in hospitalization and may incur increased costs for healthcare systems.
Based in Lund, Sweden, Europe, BrainCool AB (publ) (Spotlight markets BRAIN) is a publicly traded medical device company focused on next-generation temperature management systems. The company has two business units, braincooling, with the two CE marked products BrainCool System and RhinoChill, and in oncology with the Cooral System. BrainCool Inc., the U.S. subsidiary of BrainCool AB, is based in Annapolis, Md. The IQool System, which received FDA 510(k) clearance in 2018, was the first BrainCool product to be marketed in the United States.