U.S. Food and Drug Administration12.16.20
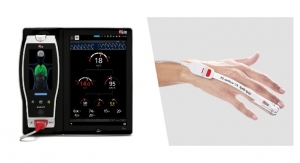
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first over-the-counter (OTC) fully at-home diagnostic test for COVID-19. The Ellume COVID-19 Home Test is a rapid, lateral flow antigen test, a type of test that runs a liquid sample along a surface with reactive molecules. The test detects fragments of proteins of the SARS-CoV-2 virus from a nasal swab sample from any individual 2 years of age or older.
“Today’s authorization is a major milestone in diagnostic testing for COVID-19. By authorizing a test for over- the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes,” said FDA Commissioner Stephen M. Hahn, M.D. “As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes.”
The announcement today of the first fully at-home OTC COVID-19 diagnostic test follows last month’s authorization of the first prescription COVID-19 test for home use and last week’s announcement of the first non-prescription test system, in which a lab processes the self-collected sample. The FDA has authorized more than 225 diagnostic tests for COVID-19 since the start of the pandemic, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing. The Ellume COVID-19 Home Test is the first COVID-19 test that can be used completely at home without a prescription.
“The FDA strongly supports innovation in test development and we have worked tirelessly with test developers to support the shared goal of getting more accurate and reliable tests to Americans who need them. Today is a promising step forward and we are eager to continue advancing additional innovation in COVID-19 testing that the science supports,” said Jeff Shuren, M.D., J.D., director of FDA’s Center for Devices and Radiological Health. “This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab. However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
Similar to other antigen tests, a small percentage of positive and negative results from this test may be false. Therefore, for patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible. This is especially true if there are fewer infections in a particular community, as false positive results can be more common when antigen tests are used in populations where there is little COVID-19 (low prevalence).
The FDA reminds patients that all tests can experience false negative and false positive results. Individuals with positive results should self-isolate and seek additional care from their health care provider. Individuals who test negative and experience COVID-like symptoms should follow up with their health care provider as negative results do not preclude an individual from SARS-CoV-2 infection.
The Ellume COVID-19 Home Test uses a mid-turbinate nasal swab (sample is collected further back than the usual nasal swab, but not as far back as nasopharyngeal swabs, which are only appropriate for use by a trained health care provider) to detect certain proteins of the virus known as antigens. The Ellume COVID-19 Home Test correctly identified 96 percent of positive samples and 100 percent of negative samples in individuals with symptoms. In people without symptoms, the test correctly identified 91 percent of positive samples and 96 percent of negative samples. The Ellume COVID-19 Home Test uses an analyzer that connects with a software application on a smartphone to help users perform the test and interpret results. Results are delivered in as little as 20 minutes to individuals via their smartphone. The mobile application requires individuals to input their zip code and date of birth, with optional fields including name and e-mail address, and reports the results as appropriate to public health authorities to monitor disease prevalence. Ellume expects to produce more than three million tests in January 2021.
The FDA continues to work with test developers to expand access to COVID-19 testing and supports further development of COVID-19 tests that can be used completely at home.
“Today’s authorization is a major milestone in diagnostic testing for COVID-19. By authorizing a test for over- the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes,” said FDA Commissioner Stephen M. Hahn, M.D. “As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes.”
The announcement today of the first fully at-home OTC COVID-19 diagnostic test follows last month’s authorization of the first prescription COVID-19 test for home use and last week’s announcement of the first non-prescription test system, in which a lab processes the self-collected sample. The FDA has authorized more than 225 diagnostic tests for COVID-19 since the start of the pandemic, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing. The Ellume COVID-19 Home Test is the first COVID-19 test that can be used completely at home without a prescription.
“The FDA strongly supports innovation in test development and we have worked tirelessly with test developers to support the shared goal of getting more accurate and reliable tests to Americans who need them. Today is a promising step forward and we are eager to continue advancing additional innovation in COVID-19 testing that the science supports,” said Jeff Shuren, M.D., J.D., director of FDA’s Center for Devices and Radiological Health. “This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab. However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
Similar to other antigen tests, a small percentage of positive and negative results from this test may be false. Therefore, for patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible. This is especially true if there are fewer infections in a particular community, as false positive results can be more common when antigen tests are used in populations where there is little COVID-19 (low prevalence).
The FDA reminds patients that all tests can experience false negative and false positive results. Individuals with positive results should self-isolate and seek additional care from their health care provider. Individuals who test negative and experience COVID-like symptoms should follow up with their health care provider as negative results do not preclude an individual from SARS-CoV-2 infection.
The Ellume COVID-19 Home Test uses a mid-turbinate nasal swab (sample is collected further back than the usual nasal swab, but not as far back as nasopharyngeal swabs, which are only appropriate for use by a trained health care provider) to detect certain proteins of the virus known as antigens. The Ellume COVID-19 Home Test correctly identified 96 percent of positive samples and 100 percent of negative samples in individuals with symptoms. In people without symptoms, the test correctly identified 91 percent of positive samples and 96 percent of negative samples. The Ellume COVID-19 Home Test uses an analyzer that connects with a software application on a smartphone to help users perform the test and interpret results. Results are delivered in as little as 20 minutes to individuals via their smartphone. The mobile application requires individuals to input their zip code and date of birth, with optional fields including name and e-mail address, and reports the results as appropriate to public health authorities to monitor disease prevalence. Ellume expects to produce more than three million tests in January 2021.
The FDA continues to work with test developers to expand access to COVID-19 testing and supports further development of COVID-19 tests that can be used completely at home.