Tolomatic04.13.20
Tolomatic, a global company in linear motion technologies, has applied its expertise to develop automation solutions for manual resuscitators, which are needed worldwide to fight the COVID-19 pandemic.
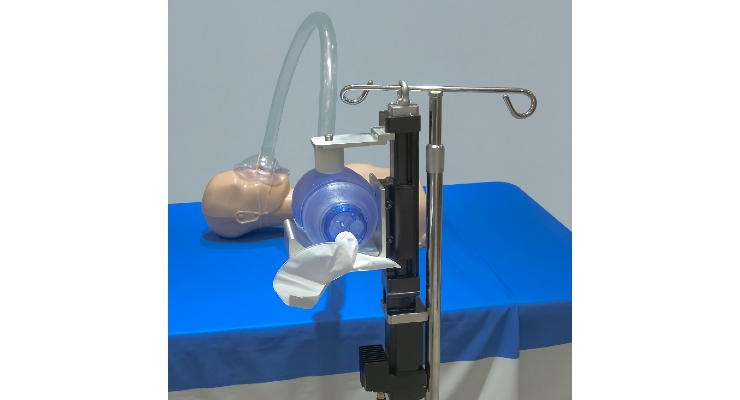
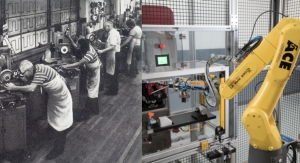
Tolomatic’s solution is a new type of ventilator that operates using an electric linear actuator. These prototypes automate a non-invasive, positive-pressure resuscitator (also self-inflating bag, bag valve mask or AMBU bag/Artificial Manual Breathing Unit). The device, used primarily in emergency situations when traditional ventilators are not available, provides oxygen to patients requiring breathing assistance. The bag valve mask is positioned over the patient’s nose and mouth. An assistant manually squeezes the bag to provide airflow via a combination of ambient air and an oxygen cylinder and connecting tube. Squeezing the bag manually is workable for short durations, but not viable for longer term care. It can also create air flow inconsistencies, as well as require extra time and labor to stabilize the patient. Tolomatic’s approach is to automate this traditionally manual process using their electric linear actuators and ensure patients would continue to get air for days or weeks.
Tolomatic’s hope is to spark interest and conversation with potential partners in developing a final design solution that can be submitted for approval.
Tolomatic screw-driven linear actuators convert rotary power from a servo motor into linear motion. This provides smooth and consistent operation of the system, allowing the device to control the velocity, the acceleration and the distance of any move at any point in time. This controlled motion allows for a more continuous volume of air per compression cycle and a more typical breathing cycle.
Ambu bags with an automated single-direction camming solution or pneumatic actuator do not allow for varying the stroke length; only the breath frequency. As a result, the tidal volume of air flow to the patient is always the same, and the motion profile is counter to a typical breath cycle.
In contrast, linear actuator systems can change the frequency of the induced respirations and also the volume, which is not possible with fixed displacement rotary devices. The total control of motion from a linear electric actuator allows for much more flexible air flow that could now be modified for a patient’s age, size or current needs.
This solution offers reliable long life, and has the ability to integrate in alarm features in case of motor faults, or possible sensors around the airflow quantity and quality.
“We are not a medical device manufacturer and this design has not been approved by any regulatory bodies,” said Andy Zaske, vice president of sales and marketing, Tolomatic. “However, many of our customers are, and our hope is that this might spark some interest in partnering with us on an approved final design solution. We stand ready to offer our motion control expertise to help solve critical challenges during this time. Contact us for assistance with your applications. Together we will make it happen!”
Tolomatic’s solution is a new type of ventilator that operates using an electric linear actuator. These prototypes automate a non-invasive, positive-pressure resuscitator (also self-inflating bag, bag valve mask or AMBU bag/Artificial Manual Breathing Unit). The device, used primarily in emergency situations when traditional ventilators are not available, provides oxygen to patients requiring breathing assistance. The bag valve mask is positioned over the patient’s nose and mouth. An assistant manually squeezes the bag to provide airflow via a combination of ambient air and an oxygen cylinder and connecting tube. Squeezing the bag manually is workable for short durations, but not viable for longer term care. It can also create air flow inconsistencies, as well as require extra time and labor to stabilize the patient. Tolomatic’s approach is to automate this traditionally manual process using their electric linear actuators and ensure patients would continue to get air for days or weeks.
Tolomatic’s hope is to spark interest and conversation with potential partners in developing a final design solution that can be submitted for approval.
Tolomatic screw-driven linear actuators convert rotary power from a servo motor into linear motion. This provides smooth and consistent operation of the system, allowing the device to control the velocity, the acceleration and the distance of any move at any point in time. This controlled motion allows for a more continuous volume of air per compression cycle and a more typical breathing cycle.
Ambu bags with an automated single-direction camming solution or pneumatic actuator do not allow for varying the stroke length; only the breath frequency. As a result, the tidal volume of air flow to the patient is always the same, and the motion profile is counter to a typical breath cycle.
In contrast, linear actuator systems can change the frequency of the induced respirations and also the volume, which is not possible with fixed displacement rotary devices. The total control of motion from a linear electric actuator allows for much more flexible air flow that could now be modified for a patient’s age, size or current needs.
This solution offers reliable long life, and has the ability to integrate in alarm features in case of motor faults, or possible sensors around the airflow quantity and quality.
“We are not a medical device manufacturer and this design has not been approved by any regulatory bodies,” said Andy Zaske, vice president of sales and marketing, Tolomatic. “However, many of our customers are, and our hope is that this might spark some interest in partnering with us on an approved final design solution. We stand ready to offer our motion control expertise to help solve critical challenges during this time. Contact us for assistance with your applications. Together we will make it happen!”