Prisma Health04.06.20
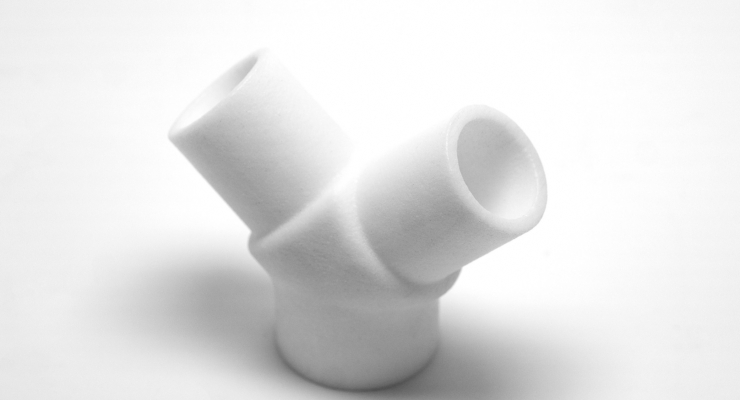
Prisma Health is collaborating with Ethicon Inc., part of the Johnson & Johnson Medical Devices Companies, to make and distribute a ventilator expansion device, called the VESper Ventilator Expansion Splitter. The VESper Ventilator Expansion Splitter is authorized for emergency use only to allow a single ventilator to be fitted with the Ventilator Splitter to be used for two rescuable patients for ventilatory support during the COVID-19 pandemic until individual ventilators are available.
Due to the predicted dire ventilator shortage, Prisma Health received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the device in response to the urgency for more ventilators as a result of the pandemic.
Using 3D printing manufacturing technology, Ethicon will manufacture and distribute the VESper Ventilator Expansion Splitter at no cost to healthcare providers in the United States under the EUA during the current COVID-19 pandemic. The initial distribution of the device will be limited to the United States as Prisma Health and Ethicon are developing strategies to navigate various regulatory pathways and fulfill orders worldwide.
"Our goal is to provide healthcare providers with an emergency use device for critical patients in rapid time at no cost," said Mark O'Halla, Prisma Health president and chief executive officer. "We are pleased to announce this collaboration with Ethicon which brings nationwide scale and expertise for the manufacturing and distribution of VESper by a top-tier medical device company."
Prisma Health experts are working with national COVID-19 teams, healthcare providers and state and local health authorities to identify healthcare providers where emergency use of the device is needed and will be working closely with these teams to monitor clinical outcomes. Ethicon is bringing its 3D printing manufacturing and supply chain expertise to meet the challenging demand of providing the ventilator expansion splitters.
Healthcare providers interested in the device should complete the request form on www.PrismaHealth.org/VESper. Prisma Health will review all requests so that the device can be prioritized to areas and hospitals with the greatest need. The VESper Ventilator Expansion Splitter will be packaged a dozen to a box, two required for each patient in accordance with the Instructions for Use that includes the clinical protocol as published online on March 31, 2020 by the U.S. Department of Health & Human Services Assistant Secretary and the U.S Surgeon General titled "Optimizing Ventilator Use During the COVID-19 Pandemic." The protocol was included in Appendix D: Ventilator Sharing Protocol: Dual-Patient Ventilation with a Single Mechanical Ventilator for Use during Critical Ventilator Shortage. It is critical that this device be used by only trained healthcare providers with relevant expertise where it is deemed clinically appropriate and necessary and only to address the COVID-19 health emergency.
"This collaboration will allow us to have the highest VESper device consistency for healthcare providers in need of treating COVID-19 patients in the United States and beyond," said Peter Tilkemeier, M.D., chair, Department of Medicine, Prisma Health–Upstate. "We are providing an option to the medical community, reviewed and authorized by the FDA. Each hospital and community needs to decide the best option for them as they begin experiencing ventilator shortages due to COVID-19."
"Bringing this device to fruition took a village," said Marjorie Jenkins, M.D., chief academic officer for Prisma Health–Upstate and dean of the University of South Carolina School of Medicine Greenville. "In addition to our founding team of inventors, collaborators and university partners—Clemson University and the University of South Carolina—our sincere compliments to the many, many industry partners who stepped forward during this public health crisis to support us, including Ethicon; Hewlett-Packard Inc. and its Digital Manufacturing Network; Salesforce; AzimuthDS; the Sargent Foundation; and, the FDA Center for Devices and Radiological Health."
Due to the predicted dire ventilator shortage, Prisma Health received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the device in response to the urgency for more ventilators as a result of the pandemic.
Using 3D printing manufacturing technology, Ethicon will manufacture and distribute the VESper Ventilator Expansion Splitter at no cost to healthcare providers in the United States under the EUA during the current COVID-19 pandemic. The initial distribution of the device will be limited to the United States as Prisma Health and Ethicon are developing strategies to navigate various regulatory pathways and fulfill orders worldwide.
"Our goal is to provide healthcare providers with an emergency use device for critical patients in rapid time at no cost," said Mark O'Halla, Prisma Health president and chief executive officer. "We are pleased to announce this collaboration with Ethicon which brings nationwide scale and expertise for the manufacturing and distribution of VESper by a top-tier medical device company."
Prisma Health experts are working with national COVID-19 teams, healthcare providers and state and local health authorities to identify healthcare providers where emergency use of the device is needed and will be working closely with these teams to monitor clinical outcomes. Ethicon is bringing its 3D printing manufacturing and supply chain expertise to meet the challenging demand of providing the ventilator expansion splitters.
Healthcare providers interested in the device should complete the request form on www.PrismaHealth.org/VESper. Prisma Health will review all requests so that the device can be prioritized to areas and hospitals with the greatest need. The VESper Ventilator Expansion Splitter will be packaged a dozen to a box, two required for each patient in accordance with the Instructions for Use that includes the clinical protocol as published online on March 31, 2020 by the U.S. Department of Health & Human Services Assistant Secretary and the U.S Surgeon General titled "Optimizing Ventilator Use During the COVID-19 Pandemic." The protocol was included in Appendix D: Ventilator Sharing Protocol: Dual-Patient Ventilation with a Single Mechanical Ventilator for Use during Critical Ventilator Shortage. It is critical that this device be used by only trained healthcare providers with relevant expertise where it is deemed clinically appropriate and necessary and only to address the COVID-19 health emergency.
"This collaboration will allow us to have the highest VESper device consistency for healthcare providers in need of treating COVID-19 patients in the United States and beyond," said Peter Tilkemeier, M.D., chair, Department of Medicine, Prisma Health–Upstate. "We are providing an option to the medical community, reviewed and authorized by the FDA. Each hospital and community needs to decide the best option for them as they begin experiencing ventilator shortages due to COVID-19."
"Bringing this device to fruition took a village," said Marjorie Jenkins, M.D., chief academic officer for Prisma Health–Upstate and dean of the University of South Carolina School of Medicine Greenville. "In addition to our founding team of inventors, collaborators and university partners—Clemson University and the University of South Carolina—our sincere compliments to the many, many industry partners who stepped forward during this public health crisis to support us, including Ethicon; Hewlett-Packard Inc. and its Digital Manufacturing Network; Salesforce; AzimuthDS; the Sargent Foundation; and, the FDA Center for Devices and Radiological Health."