Business Wire01.09.20
Reflow Medical announces that the Temporary Spur Stent System, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral artery disease, has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA).
The Breakthrough Devices Program is designed to give patients and health care providers timely access to medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The program offers Reflow Medical the opportunity to interact with experts at the FDA throughout the premarket review phase, in order to help speed the development, assessment and review of the device.
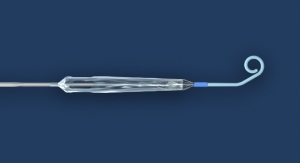
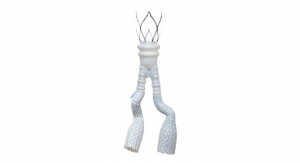
The Temporary Spur Stent System is a novel combination device consisting of a patented retrievable stent system having a series of radially expandable spikes designed to create multiple pathways to deliver antiproliferative drugs for increased uptake into the vessel wall and facilitate acute luminal gain, without leaving anything behind. The device was developed in response to unmet clinical needs resulting in high rates of restenosis and treatment challenges in patients with BTK disease.
“We are extremely grateful to the FDA for their expedited designation of the Temporary Spur Stent System as a Breakthrough Device. We plan to take full advantage of the Program’s benefits, accelerating our efforts towards meeting the requirements of the review process as we advance this novel technology, with the goal of improving the lives of patients,” says Isa Rizk, CEO of Reflow Medical.
As Reflow continues to build on clinical evidence supporting the Temporary Spur Stent System, the company looks forward to furthering development in other clinical areas, based on the Spur technology platform.
The Breakthrough Devices Program is designed to give patients and health care providers timely access to medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The program offers Reflow Medical the opportunity to interact with experts at the FDA throughout the premarket review phase, in order to help speed the development, assessment and review of the device.
The Temporary Spur Stent System is a novel combination device consisting of a patented retrievable stent system having a series of radially expandable spikes designed to create multiple pathways to deliver antiproliferative drugs for increased uptake into the vessel wall and facilitate acute luminal gain, without leaving anything behind. The device was developed in response to unmet clinical needs resulting in high rates of restenosis and treatment challenges in patients with BTK disease.
“We are extremely grateful to the FDA for their expedited designation of the Temporary Spur Stent System as a Breakthrough Device. We plan to take full advantage of the Program’s benefits, accelerating our efforts towards meeting the requirements of the review process as we advance this novel technology, with the goal of improving the lives of patients,” says Isa Rizk, CEO of Reflow Medical.
As Reflow continues to build on clinical evidence supporting the Temporary Spur Stent System, the company looks forward to furthering development in other clinical areas, based on the Spur technology platform.