Business Wire12.16.19
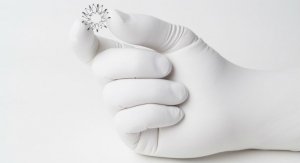
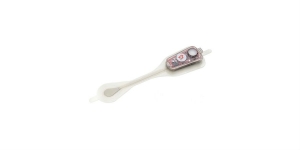
PQ Bypass Inc., a clinical-stage medical device company, has enrolled the first patient in its TORUS 2 multi-center clinical trial of its self-expanding TORUS stent graft system, a novel technology designed for the treatment of peripheral artery disease (PAD) in the superficial femoral artery (SFA). This inaugural enrollment comes less than a month after the study received unconditional approval from the U.S. Food and Drug Administration for the TORUS 2 original Investigative Device Exemption.
The TORUS 2 study (The PQ Bypass pivOtal IDE intra-aRterial stent graft study for occlUsive and re-Stenotic fem-pop revascularization) is a prospective, single-arm trial of 188 patients at up to 40 sites. The study is led by national co-principal investigators Peter Schneider M.D., professor of Surgery, Division of Vascular and Endovascular Surgery at University of California San Francisco, and Ehrin Armstrong M.D., MSc, FSCAI, director of Interventional Cardiology at the Rocky Mountain Regional VA Hospital and professor of Cardiology at University of Colorado School of Medicine.
The objective of the trial is to evaluate the safety and effectiveness of the TORUS Stent Graft System in the treatment of obstructive atherosclerotic lesions of the superficial femoral and/or proximal popliteal arteries. The TORUS Stent Graft System received CE Mark in 2017 for use in the DETOUR percutaneous femoropopliteal bypass procedure, based on the DETOUR 1 global study. The technology has also been evaluated in the TORUS 1 study in Europe and is currently under evaluation in the DETOUR 2 IDE for percutaneous femoropopliteal bypass in the United States and Europe.
"There is an unmet need in our care of PAD patients for an updated, optimized, and specifically designed stent graft for SFA-popliteal use,” Dr. Schneider said. “I believe this technology could become an important part of our endovascular armamentarium."
Dr. Armstrong added, "This study is intended to further the growing body of evidence for the TORUS stent graft. I am pleased to be involved in such an important step forward in the percutaneous treatment of PAD.”
Vaqar Ali M.D., FACC, FSCAI, vice president and Cath Lab director at First Coast Cardiovascular Institute in Jacksonville, Fla., enrolled and treated the trial's first patient.
"Our patient had a challenging SFA lesion that was treated thanks to the radial strength and flexibility afforded by the robust design of the TORUS stent graft," Dr. Ali said. "We are thrilled to be the first site to enroll in this important trial and to be part of bringing this technology forward as a potential option for our patients.”
PQ Bypass General Manager Heather Simonsen called the enrollment of the first patient "a significant milestone for PQ Bypass as we initiate our second pivotal IDE evaluating our technology in two different applications for patients with PAD. We’re thrilled to commence TORUS 2, continue enrollment in DETOUR 2, and look forward to working with our investigators to execute two pivotal trials.”
PQ Bypass Inc. is a Silicon Valley-based medical device company pioneering treatments for peripheral artery disease. The company’s physician-driven innovations include the TORUS Stent Graft, PQ Snare, and PQ Crossing Device. These technologies are currently being evaluated in two global, multicenter, IDE studies: DETOUR 2 and TORUS 2. DETOUR 2 is focused on SFA lesions >200 mm via a percutaneous femoropopliteal bypass procedure, and TORUS 2 is focused on SFA lesions <180 mm in length.
Crafted by world-renowned experts in the field of peripheral arterial disease, PQ Bypass devices are built upon nearly a decade of research and patented technological advancement. PQ Bypass is recognized by MedTech Outlook Magazine as one of the Top 10 Cardiovascular Companies of 2019 and earned Frost and Sullivan’s European Technology Innovation Award for the DETOUR procedure in 2017.
The TORUS stent graft is limited by federal law to investigational use only and is not available for sale in the United States.
The TORUS 2 study (The PQ Bypass pivOtal IDE intra-aRterial stent graft study for occlUsive and re-Stenotic fem-pop revascularization) is a prospective, single-arm trial of 188 patients at up to 40 sites. The study is led by national co-principal investigators Peter Schneider M.D., professor of Surgery, Division of Vascular and Endovascular Surgery at University of California San Francisco, and Ehrin Armstrong M.D., MSc, FSCAI, director of Interventional Cardiology at the Rocky Mountain Regional VA Hospital and professor of Cardiology at University of Colorado School of Medicine.
The objective of the trial is to evaluate the safety and effectiveness of the TORUS Stent Graft System in the treatment of obstructive atherosclerotic lesions of the superficial femoral and/or proximal popliteal arteries. The TORUS Stent Graft System received CE Mark in 2017 for use in the DETOUR percutaneous femoropopliteal bypass procedure, based on the DETOUR 1 global study. The technology has also been evaluated in the TORUS 1 study in Europe and is currently under evaluation in the DETOUR 2 IDE for percutaneous femoropopliteal bypass in the United States and Europe.
"There is an unmet need in our care of PAD patients for an updated, optimized, and specifically designed stent graft for SFA-popliteal use,” Dr. Schneider said. “I believe this technology could become an important part of our endovascular armamentarium."
Dr. Armstrong added, "This study is intended to further the growing body of evidence for the TORUS stent graft. I am pleased to be involved in such an important step forward in the percutaneous treatment of PAD.”
Vaqar Ali M.D., FACC, FSCAI, vice president and Cath Lab director at First Coast Cardiovascular Institute in Jacksonville, Fla., enrolled and treated the trial's first patient.
"Our patient had a challenging SFA lesion that was treated thanks to the radial strength and flexibility afforded by the robust design of the TORUS stent graft," Dr. Ali said. "We are thrilled to be the first site to enroll in this important trial and to be part of bringing this technology forward as a potential option for our patients.”
PQ Bypass General Manager Heather Simonsen called the enrollment of the first patient "a significant milestone for PQ Bypass as we initiate our second pivotal IDE evaluating our technology in two different applications for patients with PAD. We’re thrilled to commence TORUS 2, continue enrollment in DETOUR 2, and look forward to working with our investigators to execute two pivotal trials.”
PQ Bypass Inc. is a Silicon Valley-based medical device company pioneering treatments for peripheral artery disease. The company’s physician-driven innovations include the TORUS Stent Graft, PQ Snare, and PQ Crossing Device. These technologies are currently being evaluated in two global, multicenter, IDE studies: DETOUR 2 and TORUS 2. DETOUR 2 is focused on SFA lesions >200 mm via a percutaneous femoropopliteal bypass procedure, and TORUS 2 is focused on SFA lesions <180 mm in length.
Crafted by world-renowned experts in the field of peripheral arterial disease, PQ Bypass devices are built upon nearly a decade of research and patented technological advancement. PQ Bypass is recognized by MedTech Outlook Magazine as one of the Top 10 Cardiovascular Companies of 2019 and earned Frost and Sullivan’s European Technology Innovation Award for the DETOUR procedure in 2017.
The TORUS stent graft is limited by federal law to investigational use only and is not available for sale in the United States.