Johnson & Johnson Medical Devices Companies12.03.19
Many patients today are entering the operating room with multiple factors that can cause disruptive bleeding during surgery, which can be challenging to manage and potentially dangerous. To help address this, Johnson & Johnson Medical Devices Companies* announced today that Ethicon** has launched VISTASEAL Fibrin Sealant (Human) to help surgeons manage bleeding during surgery.
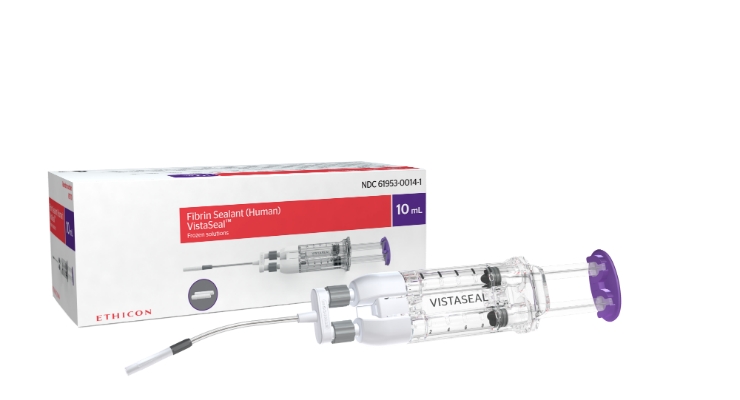
VISTASEAL Fibrin Sealant (Human) contains a combination of fibrinogen and thrombin, clotting proteins found in human plasma. When applied to the bleeding site, it forms a rapid, adherent, and durable cloti and has been demonstrated to sustain hemostasis (stoppage of bleeding), even in high-risk patients.i,ii,iii In three randomized controlled trials, VISTASEAL was associated with a lower overall hemostatic retreatment rate (0.9 percent to 7.8 percent) compared to standard treatments (8 percent to 16.7 percent).iv
"Surgical patients often present with multiple risk factors such as coagulopathies, uncontrolled diabetes, renal or liver failure or are taking anticoagulants or chronic antiplatelet therapies that may interfere with the body's natural ability to form a clot, which increases the risk of surgical bleeding," said David Kwon, M.D., FACS, director of Surgical Oncology, Henry Ford Hospital Ɨ. "VISTASEAL has the potential to offer a rapid, adherent, durable clot even in my most demanding cases."
VISTASEAL is the first innovation to emerge from Ethicon's strategic partnership with plasma industry leader Grifols, which developed the VISTASEAL Fibrin Sealant (Human) and licensed it to Ethicon. The collaboration combines Ethicon's expertise in developing device technology with Grifols' ability to produce critical plasma-based therapies.
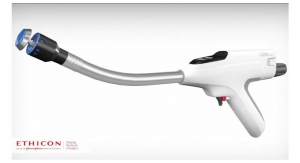
VISTASEAL is the first fibrin sealant exclusively designed to be sprayed without gas in both open and minimally invasive procedures, and it comes in pre-filled syringes. This eliminates some of the steps required for set-up with gas and may save valuable time in the operating room. The VISTASEAL Dual Applicator tip is also uniquely malleable enabling access to difficult anatomy and enhanced spray control.v,vi,±
Throughout a surgical procedure, bleeding must be controlled not only to provide a clear view of the operative site, but also to prevent the adverse clinical outcomes associated with blood loss. An estimated 32 to 68 percent of cases in open surgery procedures experience disruptive bleeding events.vii
VISTASEAL is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) are ineffective or impractical. VISTASEAL is effective in patients treated with heparin (blood thinner).
"VISTASEAL is the latest addition to our broad portfolio of primary and adjunctive hemostat solutions that address the growing and wide-ranging challenges surgeons face in managing bleeding," said Oray Boston, president, Global Biosurgery, Ethicon. "We will continuously aim to advance the standard of care in surgery to better serve health care professionals and improve outcomes for patients."
Ethicon, part of Johnson & Johnson Medical Devices Companies, has made significant contributions to surgery for more than 100 years from creating the first sutures, to revolutionizing surgery with minimally invasive procedures. Its continuing dedication to Shape the Future of Surgery is built on our commitment to help address the world's most pressing health care issues and improve and save more lives. Through Ethicon's surgical technologies and solutions including sutures, staplers, energy devices, trocars and hemostats and its commitment to treat serious medical conditions like obesity worldwide, the company delivers innovation to make a life-changing impact.
Johnson & Johnson Medical Devices Companies helps people live their best lives. Building on more than a century of expertise, the company tackles pressing healthcare challenges, and takes bold steps that lead to new standards of care while improving people's healthcare experiences. In surgery, orthopedics, vision and interventional solutions, Johnson & Johnson Medical Devices Companies helps save lives and paves the way to a healthier future for everyone, everywhere.
VISTASEAL is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. VISTASEAL is effective in heparinized patients.
* The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's Medical Devices segment.
** Ethicon represents the products and services of Ethicon Inc., Ethicon Endo-Surgery LLC and certain of their affiliates. All other trademarks are the property of their respective owners.
*** Based on number of set-up steps and thawing time required for VISTASEALTM Dual Applicator vs competition.
Ɨ Dr. Kwon is a paid consultant for Ethicon
± Compared to gas‐assisted spray
References
i Bjelovic M, Ayguasonosa J, Kim RD, et al. A prospective, randomized, phase III study to evaluate the efficacy and safety of fibrin sealant Grifols as an adjunct to hemostasis as compared to cellulose sheets in hepatic surgery resections. J Gastrointest Surg. 2018. 22:1939-1949.
ii Chetter I, Stansby G, Sarralde JA, et al. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann Vasc Surg. 2017;45:127-137
iii Hunt BJ. Bleeding and coagulapathies in critical care. N Engl J Med. 2014;370:847-859. 4. VISTASEALTM Fibrin Sealant (Human).
iv Danker W, Ferko N, Hogan A. VISTASEAL ISPOR EU cost analysis. June 11, 2019. Ethicon, Inc.
v Completion Report for Angled Adherence, Study No. 100682576. December 10, 2018. Ethicon, Inc.
vi Phillips R. VISTASEAL ASA Design Verification Memo for Expression Force and Surface Area. 100675646 Rev 1. December 5, 2018. Ethicon, Inc.
vii Corral M, Hollmann S, Ferko N, Broder M, Chang E, Sun G. Health and Economic Consequences of Controlled vs Uncontrolled Surgical Bleeding in Patients Treated with Haemostatic Agents: A Retrospective Analysis of the Premier Perspective Database. SABM, 2014.
VISTASEAL Fibrin Sealant (Human) contains a combination of fibrinogen and thrombin, clotting proteins found in human plasma. When applied to the bleeding site, it forms a rapid, adherent, and durable cloti and has been demonstrated to sustain hemostasis (stoppage of bleeding), even in high-risk patients.i,ii,iii In three randomized controlled trials, VISTASEAL was associated with a lower overall hemostatic retreatment rate (0.9 percent to 7.8 percent) compared to standard treatments (8 percent to 16.7 percent).iv
"Surgical patients often present with multiple risk factors such as coagulopathies, uncontrolled diabetes, renal or liver failure or are taking anticoagulants or chronic antiplatelet therapies that may interfere with the body's natural ability to form a clot, which increases the risk of surgical bleeding," said David Kwon, M.D., FACS, director of Surgical Oncology, Henry Ford Hospital Ɨ. "VISTASEAL has the potential to offer a rapid, adherent, durable clot even in my most demanding cases."
VISTASEAL is the first innovation to emerge from Ethicon's strategic partnership with plasma industry leader Grifols, which developed the VISTASEAL Fibrin Sealant (Human) and licensed it to Ethicon. The collaboration combines Ethicon's expertise in developing device technology with Grifols' ability to produce critical plasma-based therapies.
VISTASEAL is the first fibrin sealant exclusively designed to be sprayed without gas in both open and minimally invasive procedures, and it comes in pre-filled syringes. This eliminates some of the steps required for set-up with gas and may save valuable time in the operating room. The VISTASEAL Dual Applicator tip is also uniquely malleable enabling access to difficult anatomy and enhanced spray control.v,vi,±
Throughout a surgical procedure, bleeding must be controlled not only to provide a clear view of the operative site, but also to prevent the adverse clinical outcomes associated with blood loss. An estimated 32 to 68 percent of cases in open surgery procedures experience disruptive bleeding events.vii
VISTASEAL is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) are ineffective or impractical. VISTASEAL is effective in patients treated with heparin (blood thinner).
"VISTASEAL is the latest addition to our broad portfolio of primary and adjunctive hemostat solutions that address the growing and wide-ranging challenges surgeons face in managing bleeding," said Oray Boston, president, Global Biosurgery, Ethicon. "We will continuously aim to advance the standard of care in surgery to better serve health care professionals and improve outcomes for patients."
Ethicon, part of Johnson & Johnson Medical Devices Companies, has made significant contributions to surgery for more than 100 years from creating the first sutures, to revolutionizing surgery with minimally invasive procedures. Its continuing dedication to Shape the Future of Surgery is built on our commitment to help address the world's most pressing health care issues and improve and save more lives. Through Ethicon's surgical technologies and solutions including sutures, staplers, energy devices, trocars and hemostats and its commitment to treat serious medical conditions like obesity worldwide, the company delivers innovation to make a life-changing impact.
Johnson & Johnson Medical Devices Companies helps people live their best lives. Building on more than a century of expertise, the company tackles pressing healthcare challenges, and takes bold steps that lead to new standards of care while improving people's healthcare experiences. In surgery, orthopedics, vision and interventional solutions, Johnson & Johnson Medical Devices Companies helps save lives and paves the way to a healthier future for everyone, everywhere.
VISTASEAL is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. VISTASEAL is effective in heparinized patients.
* The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's Medical Devices segment.
** Ethicon represents the products and services of Ethicon Inc., Ethicon Endo-Surgery LLC and certain of their affiliates. All other trademarks are the property of their respective owners.
*** Based on number of set-up steps and thawing time required for VISTASEALTM Dual Applicator vs competition.
Ɨ Dr. Kwon is a paid consultant for Ethicon
± Compared to gas‐assisted spray
References
i Bjelovic M, Ayguasonosa J, Kim RD, et al. A prospective, randomized, phase III study to evaluate the efficacy and safety of fibrin sealant Grifols as an adjunct to hemostasis as compared to cellulose sheets in hepatic surgery resections. J Gastrointest Surg. 2018. 22:1939-1949.
ii Chetter I, Stansby G, Sarralde JA, et al. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann Vasc Surg. 2017;45:127-137
iii Hunt BJ. Bleeding and coagulapathies in critical care. N Engl J Med. 2014;370:847-859. 4. VISTASEALTM Fibrin Sealant (Human).
iv Danker W, Ferko N, Hogan A. VISTASEAL ISPOR EU cost analysis. June 11, 2019. Ethicon, Inc.
v Completion Report for Angled Adherence, Study No. 100682576. December 10, 2018. Ethicon, Inc.
vi Phillips R. VISTASEAL ASA Design Verification Memo for Expression Force and Surface Area. 100675646 Rev 1. December 5, 2018. Ethicon, Inc.
vii Corral M, Hollmann S, Ferko N, Broder M, Chang E, Sun G. Health and Economic Consequences of Controlled vs Uncontrolled Surgical Bleeding in Patients Treated with Haemostatic Agents: A Retrospective Analysis of the Premier Perspective Database. SABM, 2014.