Business Wire11.20.19
Penumbra Inc., a global healthcare company focused on innovative therapies, has enrolled the first patient into the CHEETAH study, a prospective, multi-center U.S. study to evaluate the safety and performance of the Indigo System with CAT RX Aspiration Catheter in the coronary vessels. Introduced in 2018, as part of the Indigo Aspiration System, the Indigo CAT RX Aspiration Catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature.
“This important study will help inform us of the potential impact of clot removal in patients with high thrombus burden,” said Larry J. Diaz-Sandoval, M.D., director, endovascular research and interventions, Metro Health-University of Michigan Health Hospital, Grand Rapids, Mich., whose team enrolled the first patient in the CHEETAH study.
The Indigo System CAT RX device uses mechanical power aspiration to remove thrombus in the coronaries. The post-market, prospective CHEETAH study will enroll up to 400 patients presenting with coronary thrombus who are referred for percutaneous coronary intervention (PCI) at up to 25 U.S. centers (ClinicalTrials.gov Identifier: NCT03957473). The primary study endpoint is a composition of cardiovascular (CV) death, recurrent myocardial infarction (MI), cardiogenic shock or new or worsening New York Heart Association (NYHA) Class IV heart failure within 30 days. Secondary endpoints include final TIMI flow grade, final TIMI thrombus grade, and safety assessments at six months.
“Penumbra has adapted over 10 years of neuro thrombectomy experience to address the limitations of traditional manual aspiration for the coronaries by development of the Indigo System CAT RX device,” said national principal investigator S. Jay Mathews, M.D., director, cardiac catheterization laboratory, Manatee Memorial Hospital in Bradenton, Fla. “This significant upgrade in innovation from syringe-based aspiration to mechanical power aspiration coupled with highly trackable catheter technology has enabled us to improve our door to reperfusion times and thereby to improve patient care.”
“Just like the IMS-III trial for ischemic stroke, the TOTAL trial highlighted that new therapies are needed to improve the outcomes of these patients with high thrombus burden,” said Jasvindar Singh, M.D., associate professor of medicine and director of cardiac catheterization laboratory at Barnes Jewish Hospital/ Washington University School of Medicine, St. Louis. “The CHEETAH study is the first step toward making coronary mechanical thrombectomy standard of care for high thrombus burden patients. We expect this study to refine our technique of thrombus aspiration and better understand the utilization of CAT RX for patients with high thrombus burden.”
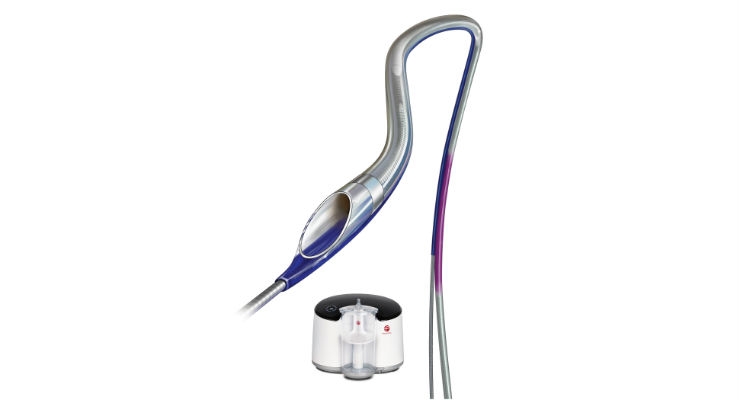
In 2014, Penumbra introduced the Indigo System, a continuous aspiration mechanical thrombectomy system designed to remove clot from arteries and veins in the peripheral vasculature. The Indigo System utilizes the Penumbra ENGINE aspiration source to deliver nearly pure, continuous vacuum suction to the Indigo System Aspiration Catheters to address thrombus in vessels of various sizes. The Indigo System CAT RX Aspiration Catheter was introduced in 2018. As part of the Indigo System, the Indigo CAT RX Aspiration Catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature. The Indigo CAT RX is a 6-French compatible, rapid exchange catheter designed for maximized clot removal with its large aspiration lumen and engineered for advanced trackability and deliverability as a result of seven material transitions and a proximal laser cut hypotube. The proprietary Separator 4 may be used together with the Indigo CAT RX for mechanical clot engagement.
Penumbra Inc., headquartered in Alameda, Calif., is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures, and markets products and has a broad portfolio that addresses challenging medical conditions and significant clinical needs across two major markets, neuro and vascular. Penumbra sells its products to hospitals primarily through its direct sales organization in the United States, most of Europe, Canada and Australia, and through distributors in select international markets.
“This important study will help inform us of the potential impact of clot removal in patients with high thrombus burden,” said Larry J. Diaz-Sandoval, M.D., director, endovascular research and interventions, Metro Health-University of Michigan Health Hospital, Grand Rapids, Mich., whose team enrolled the first patient in the CHEETAH study.
The Indigo System CAT RX device uses mechanical power aspiration to remove thrombus in the coronaries. The post-market, prospective CHEETAH study will enroll up to 400 patients presenting with coronary thrombus who are referred for percutaneous coronary intervention (PCI) at up to 25 U.S. centers (ClinicalTrials.gov Identifier: NCT03957473). The primary study endpoint is a composition of cardiovascular (CV) death, recurrent myocardial infarction (MI), cardiogenic shock or new or worsening New York Heart Association (NYHA) Class IV heart failure within 30 days. Secondary endpoints include final TIMI flow grade, final TIMI thrombus grade, and safety assessments at six months.
“Penumbra has adapted over 10 years of neuro thrombectomy experience to address the limitations of traditional manual aspiration for the coronaries by development of the Indigo System CAT RX device,” said national principal investigator S. Jay Mathews, M.D., director, cardiac catheterization laboratory, Manatee Memorial Hospital in Bradenton, Fla. “This significant upgrade in innovation from syringe-based aspiration to mechanical power aspiration coupled with highly trackable catheter technology has enabled us to improve our door to reperfusion times and thereby to improve patient care.”
“Just like the IMS-III trial for ischemic stroke, the TOTAL trial highlighted that new therapies are needed to improve the outcomes of these patients with high thrombus burden,” said Jasvindar Singh, M.D., associate professor of medicine and director of cardiac catheterization laboratory at Barnes Jewish Hospital/ Washington University School of Medicine, St. Louis. “The CHEETAH study is the first step toward making coronary mechanical thrombectomy standard of care for high thrombus burden patients. We expect this study to refine our technique of thrombus aspiration and better understand the utilization of CAT RX for patients with high thrombus burden.”
In 2014, Penumbra introduced the Indigo System, a continuous aspiration mechanical thrombectomy system designed to remove clot from arteries and veins in the peripheral vasculature. The Indigo System utilizes the Penumbra ENGINE aspiration source to deliver nearly pure, continuous vacuum suction to the Indigo System Aspiration Catheters to address thrombus in vessels of various sizes. The Indigo System CAT RX Aspiration Catheter was introduced in 2018. As part of the Indigo System, the Indigo CAT RX Aspiration Catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature. The Indigo CAT RX is a 6-French compatible, rapid exchange catheter designed for maximized clot removal with its large aspiration lumen and engineered for advanced trackability and deliverability as a result of seven material transitions and a proximal laser cut hypotube. The proprietary Separator 4 may be used together with the Indigo CAT RX for mechanical clot engagement.
Penumbra Inc., headquartered in Alameda, Calif., is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures, and markets products and has a broad portfolio that addresses challenging medical conditions and significant clinical needs across two major markets, neuro and vascular. Penumbra sells its products to hospitals primarily through its direct sales organization in the United States, most of Europe, Canada and Australia, and through distributors in select international markets.