Flexan11.18.19
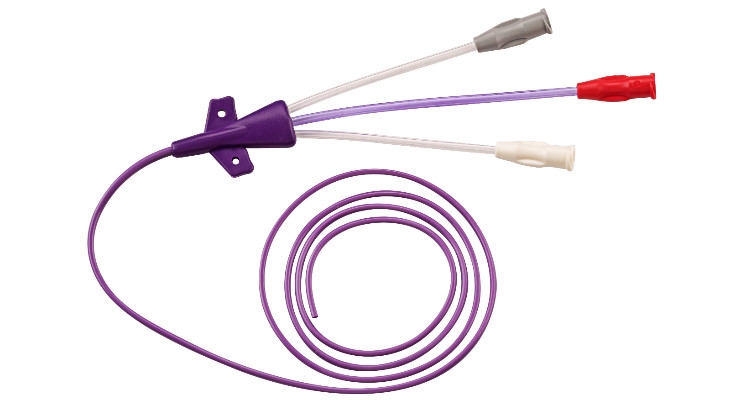
In the U.S., more than 5 million central venous catheters (CVCs) are inserted every year, which corresponds to 15 million days of treatment with CVCs. In Germany, this figure is 4.8 million in intensive care units alone. The use of these catheters is associated with some risks for the patient: the possible complications range from injured vessels and nerves to infections to pneumothoraxes, air embolisms and catheter sepsis, which can be fatal. In addition, these secondary diseases drastically increase health care costs. For this reason, efforts have long been made in medical technology to use new manufacturing methods to realize catheter designs that allow minimizing these dangers. For example, an international medical device company commissioned the experienced medical technology manufacturer Flexan to design catheters that facilitate insertion and positioning. To achieve this goal, the company chose a special urethane material that enters the body rigid but then becomes softer. This polymer was subsequently used to overmold thin-walled 5FR triple-lumen, 4FR double-lumen, and 3FR single-lumen small diameter PICCs. Flexan also designed and manufactured a 4FR catheter that has the same lumen as conventional 5FR catheters but a 14 percent smaller diameter.An overview of Flexan’s manufacturing services and processes available for the manufacture of catheters will be presented at this year’s Medica/CompaMed.
Central venous catheters are catheters that are inserted into the upper or lower vena cava—just before the right atrium of the heart—via a large vein near the heart. The application of a CVC is actually a minimally invasive procedure, but it is still possible, among other things, to perform a malpunction, perforate a vein or damage nerves in the vicinity. This can cause complications that endanger the patient's health and consequently cause an increase in treatment costs. With new manufacturing technologies for central venous catheters, standardization of insertion techniques, and the use of ultrasound guidance, however, complication rates in the U.S. have already been reduced from 11.8 to 4 to 7 percent in recent years.
Special Material and Newly Designed Catheter Tip Reduce Risk of Complications
In order to further minimize these risks with its own products, an international medical device company has commissioned Flexan’s experts to develop the critical manufacturing processes for a CVC with an optimized design that will significantly facilitate the insertion and correct positioning of its products. In support of the project, the U.S. contract manufacturer leveraged over two decades of specific PICC catheter design and manufacturing experience. For example, Flexan used a polyurethane material technology to fulfill the customer’s requirements: “We were able to mold with a urethane material that is inserted into the body in a rigid state, but immediately becomes softer there due to the body temperature,” said Eric King, V.P. and general manager at Flexan. “This plays an important role in reducing complications in catheter positioning.”
For this project, Flexan was able to draw on a broad portfolio of processes, with which biomedical components made of polymer can be adapted to the desired application. For example, Flexan is able to design and manufacture thin-walled 4FR catheters that are 14 percent smaller in diameter than conventional 5FR catheters but still have the same lumen. The company has also been able to manufacture a 4FR thin-walled catheter that has a kink resistance comparable to that of a standard 5FR catheter with the same lumen size. Moreover, the company has extensive experience in shaping catheter tips. It employs the latest high frequency technology to design the tips in a way that reduces complications during insertion.
Market Growth Opens Up High Future Potential for New Design
“Thanks to our previous experience and a very good cooperation with the client, we were able to achieve the specified project goal together,” explained King. “Within a project time of six to nine months, we succeeded in developing an optimized micro-catheter design, which significantly improves the functional performance of peripherally inserted central venous catheters.”
In addition, the Flexan team designed an efficient process control plan and inspection methods to ensure reliable binding between all vital catheter components. “This successful project for the development of a thin-walled catheter has paved the way for using the changed design for future orders. The potential is high as the global market for vascular access devices is expected to grow at an average annual rate of nearly 6.5 percent by 2023,” concluded King.
At Medica/CompaMed 2019 at Booth F20-1 in Hall 8b, company representatives will showcase Flexan’s vertically integrated custom contract manufacturing services available for the manufacture of catheters.
Central venous catheters are catheters that are inserted into the upper or lower vena cava—just before the right atrium of the heart—via a large vein near the heart. The application of a CVC is actually a minimally invasive procedure, but it is still possible, among other things, to perform a malpunction, perforate a vein or damage nerves in the vicinity. This can cause complications that endanger the patient's health and consequently cause an increase in treatment costs. With new manufacturing technologies for central venous catheters, standardization of insertion techniques, and the use of ultrasound guidance, however, complication rates in the U.S. have already been reduced from 11.8 to 4 to 7 percent in recent years.
Special Material and Newly Designed Catheter Tip Reduce Risk of Complications
In order to further minimize these risks with its own products, an international medical device company has commissioned Flexan’s experts to develop the critical manufacturing processes for a CVC with an optimized design that will significantly facilitate the insertion and correct positioning of its products. In support of the project, the U.S. contract manufacturer leveraged over two decades of specific PICC catheter design and manufacturing experience. For example, Flexan used a polyurethane material technology to fulfill the customer’s requirements: “We were able to mold with a urethane material that is inserted into the body in a rigid state, but immediately becomes softer there due to the body temperature,” said Eric King, V.P. and general manager at Flexan. “This plays an important role in reducing complications in catheter positioning.”
For this project, Flexan was able to draw on a broad portfolio of processes, with which biomedical components made of polymer can be adapted to the desired application. For example, Flexan is able to design and manufacture thin-walled 4FR catheters that are 14 percent smaller in diameter than conventional 5FR catheters but still have the same lumen. The company has also been able to manufacture a 4FR thin-walled catheter that has a kink resistance comparable to that of a standard 5FR catheter with the same lumen size. Moreover, the company has extensive experience in shaping catheter tips. It employs the latest high frequency technology to design the tips in a way that reduces complications during insertion.
Market Growth Opens Up High Future Potential for New Design
“Thanks to our previous experience and a very good cooperation with the client, we were able to achieve the specified project goal together,” explained King. “Within a project time of six to nine months, we succeeded in developing an optimized micro-catheter design, which significantly improves the functional performance of peripherally inserted central venous catheters.”
In addition, the Flexan team designed an efficient process control plan and inspection methods to ensure reliable binding between all vital catheter components. “This successful project for the development of a thin-walled catheter has paved the way for using the changed design for future orders. The potential is high as the global market for vascular access devices is expected to grow at an average annual rate of nearly 6.5 percent by 2023,” concluded King.
At Medica/CompaMed 2019 at Booth F20-1 in Hall 8b, company representatives will showcase Flexan’s vertically integrated custom contract manufacturing services available for the manufacture of catheters.