Business Wire10.24.19
Corvia Medical has sponsored and is actively enrolling patients in a heart failure (HF) device trial that, in addition to measuring traditional heart failure endpoints, includes collecting and analyzing biosensor data with physIQ’s continuous remote monitoring platform. The clinical trial is designed to evaluate the clinical efficacy of Corvia’s InterAtrial Shunt Device (IASD) in patients with heart failure and is enrolling patients at more than 100 sites worldwide. Of note, the pivotal Phase 3 study design mirrors commentary within a recent U.S. Food and Drug Administration (FDA) Public Workshop and FDA Draft Guidance for Industry related to using biosensor data.
“Heart Failure is a major global health problem and HF with preserved and mid-range ejection fraction remains a large unmet need,” said Corvia Chief Medical Officer Dr. Jan Komtebedde. “At Corvia Medical, we are evaluating a first-in-class approach to treating heart failure and, as such, see a powerful opportunity to include digital data to support IASD efficacy. It’s in this innovative spirit that we chose to partner with physIQ and bring novel real-world insights into how to assess therapeutic impact.”
In the randomized controlled double blinded study, enrolled patients are provided with a wearable biosensor and mobile data transfer hub. Each patient wears the biosensor prior to study randomization to establish a personalized pre-intervention baseline and then for up to 12 months after the device implant. Data continuously streams from the biosensor to the cloud for retrospective analysis with physIQ’s proprietary artificial intelligence-based analytics. With these real-world data and personalized analytics, the objective is to assist in demonstrating meaningful change in cardiopulmonary function and support novel clinical endpoints.
Of particular note in the REDUCE LAP HF-II study is the fact that the continuous biosensor data and artificial intelligence analytics will be generated in conjunction with the periodic evaluation of the six-minute walking distance test (6MWDT) and Kansas City Cardiomyopathy Questionnaire (KCCQ) assessments. By integrating these traditional markers of heart failure, continuous multivariate biosensor data, and hard clinical outcomes, there is extraordinary opportunity to transform how heart failure clinical trials are designed, implemented, and evaluated.
With this approach, it is possible to continuously collect physiological data and utilize artificial intelligence to assess therapeutic impact and disease progression in a way that can revolutionize how we think about clinical evidence. “Think about a race car. The driver is continuously getting actionable performance information throughout the race and using that insight to direct how to optimize the performance of the vehicle” said Matt Pipke, chief technology officer at physIQ. “However, if you applied the traditional model of healthcare to that scenario, the driver would only get information when periodically pulled over for a pit stop. It’s easy to see how that would fail; why do we accept it in healthcare? With our solution, we are developing biomarkers with life science companies that allow them to quantify clinical impact on a continuous basis and this insight can be applied in a clinical context to support regulatory submissions or to demonstrate real world evidence to payers and providers.”
The study is anticipated to complete enrollment in 2020 and, upon conclusion, will generate more than 2 million hours of continuous, annotated, clinical-level physiological data.
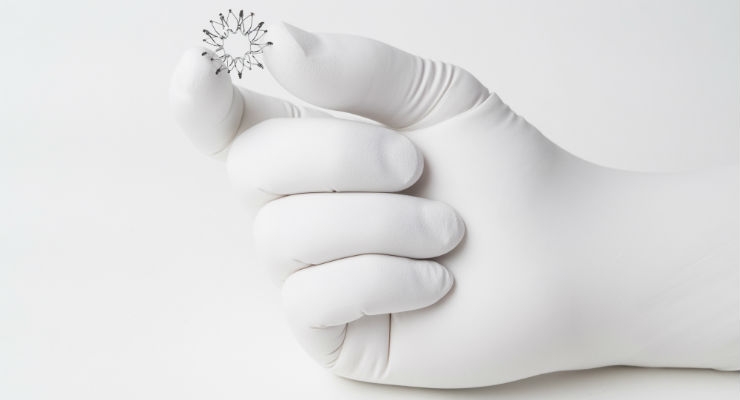
The InterAtrial Shunt Device is the world’s first transcatheter device approved in the European Union to treat heart failure with preserved (HFpEF) or mid-range ejection fraction (HFmrEF). After creating a small opening in the atrial septum, the IASD implant is deployed, forming a passage between the left and right atria that enables the left atrium to decompress at rest and during physical activity, with the aim of lowering left atrial pressure. By facilitating continuous and dynamic decompression of the left atrium, the IASD aims to improve heart failure symptoms and quality of life, decrease heart failure hospitalization rates, and reduce the overall cost burden of managing heart failure patients. The IASD is an investigational device and not available for commercial distribution in the United States.
Corvia Medical Inc. is dedicated to revolutionizing the treatment of heart failure with first-in-class transcatheter structural heart devices. Privately held, the company is backed by Third Rock Ventures, General Catalyst Partners, AccelMed, Lumira Ventures, Edwards Lifesciences, and an undisclosed strategic investor.
PhysIQ is a company dedicated to enabling proactive care delivery models through pinpointIQ, its highly scalable cloud-based platform for personalized physiology analytics. Its FDA 510(k)-cleared data analytics platform is designed to process multiple vital signs from wearable sensors to create a personalized dynamic baseline for each individual. By mapping vital sign relationships this way, physIQ’s analytics detect subtle deviations that may be a precursor to disease exacerbation or change in health. With applications in both healthcare and clinical trial support, physIQ is transforming continuous physiological data into insight for providers, health systems, payers and pharmaceutical and medical device companies.
“Heart Failure is a major global health problem and HF with preserved and mid-range ejection fraction remains a large unmet need,” said Corvia Chief Medical Officer Dr. Jan Komtebedde. “At Corvia Medical, we are evaluating a first-in-class approach to treating heart failure and, as such, see a powerful opportunity to include digital data to support IASD efficacy. It’s in this innovative spirit that we chose to partner with physIQ and bring novel real-world insights into how to assess therapeutic impact.”
In the randomized controlled double blinded study, enrolled patients are provided with a wearable biosensor and mobile data transfer hub. Each patient wears the biosensor prior to study randomization to establish a personalized pre-intervention baseline and then for up to 12 months after the device implant. Data continuously streams from the biosensor to the cloud for retrospective analysis with physIQ’s proprietary artificial intelligence-based analytics. With these real-world data and personalized analytics, the objective is to assist in demonstrating meaningful change in cardiopulmonary function and support novel clinical endpoints.
Of particular note in the REDUCE LAP HF-II study is the fact that the continuous biosensor data and artificial intelligence analytics will be generated in conjunction with the periodic evaluation of the six-minute walking distance test (6MWDT) and Kansas City Cardiomyopathy Questionnaire (KCCQ) assessments. By integrating these traditional markers of heart failure, continuous multivariate biosensor data, and hard clinical outcomes, there is extraordinary opportunity to transform how heart failure clinical trials are designed, implemented, and evaluated.
With this approach, it is possible to continuously collect physiological data and utilize artificial intelligence to assess therapeutic impact and disease progression in a way that can revolutionize how we think about clinical evidence. “Think about a race car. The driver is continuously getting actionable performance information throughout the race and using that insight to direct how to optimize the performance of the vehicle” said Matt Pipke, chief technology officer at physIQ. “However, if you applied the traditional model of healthcare to that scenario, the driver would only get information when periodically pulled over for a pit stop. It’s easy to see how that would fail; why do we accept it in healthcare? With our solution, we are developing biomarkers with life science companies that allow them to quantify clinical impact on a continuous basis and this insight can be applied in a clinical context to support regulatory submissions or to demonstrate real world evidence to payers and providers.”
The study is anticipated to complete enrollment in 2020 and, upon conclusion, will generate more than 2 million hours of continuous, annotated, clinical-level physiological data.
The InterAtrial Shunt Device is the world’s first transcatheter device approved in the European Union to treat heart failure with preserved (HFpEF) or mid-range ejection fraction (HFmrEF). After creating a small opening in the atrial septum, the IASD implant is deployed, forming a passage between the left and right atria that enables the left atrium to decompress at rest and during physical activity, with the aim of lowering left atrial pressure. By facilitating continuous and dynamic decompression of the left atrium, the IASD aims to improve heart failure symptoms and quality of life, decrease heart failure hospitalization rates, and reduce the overall cost burden of managing heart failure patients. The IASD is an investigational device and not available for commercial distribution in the United States.
Corvia Medical Inc. is dedicated to revolutionizing the treatment of heart failure with first-in-class transcatheter structural heart devices. Privately held, the company is backed by Third Rock Ventures, General Catalyst Partners, AccelMed, Lumira Ventures, Edwards Lifesciences, and an undisclosed strategic investor.
PhysIQ is a company dedicated to enabling proactive care delivery models through pinpointIQ, its highly scalable cloud-based platform for personalized physiology analytics. Its FDA 510(k)-cleared data analytics platform is designed to process multiple vital signs from wearable sensors to create a personalized dynamic baseline for each individual. By mapping vital sign relationships this way, physIQ’s analytics detect subtle deviations that may be a precursor to disease exacerbation or change in health. With applications in both healthcare and clinical trial support, physIQ is transforming continuous physiological data into insight for providers, health systems, payers and pharmaceutical and medical device companies.