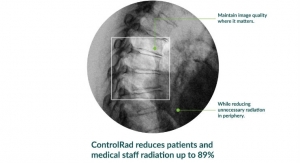
Business Wire10.10.19
STAAR Surgical Company, a developer, manufacturer, and marketer of implantable lenses and companion delivery systems for the eye, has received U.S. Food and Drug Administration (FDA) approval for a human clinical study in the United States of the EVO/EVO+ VISIAN Implantable Collamer Lens for Myopia, and EVO/EVO+ VISIAN Toric Implantable Collamer Lens for Myopia with Astigmatism.
The FDA also recommended study design modifications, which STAAR plans to incorporate into the investigational plan in a revised submission. STAAR’s current plan for the study reflects FDA’s recommendations from on-going interactive dialogue regarding the investigational protocol, including 300 subjects with a primary endpoint at six months follow up. Subjects enrolled in the trial will not undergo planned peripheral iridotomies.
“STAAR looks forward to working with FDA to incorporate the recommended protocol modifications in order for the study design to support a marketing approval submission,” said Caren Mason, president and CEO of STAAR Surgical.
Finalizing the Investigational Device Exemption (IDE) with the FDA is an important step towards the future availability of the EVO Visian ICL family of products in the United States.
STAAR Surgical Company, which has been dedicated solely to ophthalmic surgery for over 30 years, designs, develops, manufactures, and markets implantable lenses for the eye with companion delivery systems. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer Lens or “ICL,” which includes the EVO Visian ICL product line. More than 1 million Visian ICLs have been implanted to date and STAAR markets these lenses in over 75 countries. Headquartered in Lake Forest, Calif., the company operates manufacturing and packaging facilities in Aliso Viejo, Calif.; Monrovia, Calif.; and Nidau, Switzerland.
The FDA also recommended study design modifications, which STAAR plans to incorporate into the investigational plan in a revised submission. STAAR’s current plan for the study reflects FDA’s recommendations from on-going interactive dialogue regarding the investigational protocol, including 300 subjects with a primary endpoint at six months follow up. Subjects enrolled in the trial will not undergo planned peripheral iridotomies.
“STAAR looks forward to working with FDA to incorporate the recommended protocol modifications in order for the study design to support a marketing approval submission,” said Caren Mason, president and CEO of STAAR Surgical.
Finalizing the Investigational Device Exemption (IDE) with the FDA is an important step towards the future availability of the EVO Visian ICL family of products in the United States.
STAAR Surgical Company, which has been dedicated solely to ophthalmic surgery for over 30 years, designs, develops, manufactures, and markets implantable lenses for the eye with companion delivery systems. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer Lens or “ICL,” which includes the EVO Visian ICL product line. More than 1 million Visian ICLs have been implanted to date and STAAR markets these lenses in over 75 countries. Headquartered in Lake Forest, Calif., the company operates manufacturing and packaging facilities in Aliso Viejo, Calif.; Monrovia, Calif.; and Nidau, Switzerland.