PR Newswire10.08.19
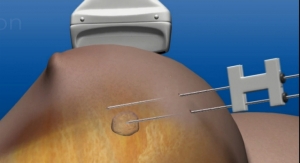
CrossBay Medical Inc. has received the CE Mark to commercialize its product, the Endometrial Tissue Sampler using CrossGlide technology, in Europe. The use of this technology allows a way to obtain an office-based endometrial biopsy, a very common tissue-sampling procedure performed in women's health offices worldwide. The ETS is the third CrossBay Medical Inc. product using the frictionless CrossGlide technology to receive marketing authorization.
In today's typical practice, medical providers need to grasp and manipulate the uterine cervix to pass a device for endometrial sampling. This often results in significant discomfort for an awake patient even with pain medication and local anesthetics. The ETS, due to the CrossGlide technology, is designed to require no grasping or manipulation of the cervix to allow for easy, dependable access into the uterine cavity regardless of the complexity or variability of the patient's specific anatomy.
There are approximately 4 million endometrial biopsies performed per year in the European Union and United States for gynecological indications, and there are an additional 2 million endometrial biopsies performed globally for infertility reasons.
"Uterine access in the office doesn't need to be difficult, nor a limit to performing procedures successfully," said Piush Vidyarthi, CEO, CrossBay Medical Inc. "The ETS using the CrossGlide technology was developed with the intent to address the typical discomfort associated with biopsy performed in the diagnostic work-up of abnormal uterine bleeding disorders and infertility. None of the procedures performed with the device required manipulation of the uterus."
Upon receipt of the CE Mark, the ETS using CrossGlide was evaluated by six doctors in Leuven, Belgium; and Naples, Italy.
"We have used the ETS on various patients and can confirm that the passage through the cervix was absolutely easy and the patient felt no pain. Endometrial sampling can be performed under ultrasound visualization and the probe remains in place until the endometrium is removed using a controlled negative pressure," said Dr. Rudi Campo from the Life Expert Centre in Leuven, Belgium.
"The ETS makes crossing the cervical canal much easier because you are not pushing anything across the cervix. Also, since the technology utilizes saline to function, the biopsy can be performed using ultrasound concurrently," said Dr. Attilio Di Spiezio Sardo from University Federico II Hospital in Naples, Italy.
The mission of CrossBay Medical is to empower physicians to deliver compassionate in-office care by offering a suite of products based on the CrossGlide technology. The company will continue to develop and commercialize medical devices using CrossGlide across a wide array of gynecological applications in the coming years.
"At CrossBay Medical, we are using the power of technology to develop products that give physicians the opportunity to bring more procedures into the office. Through our patient evaluations, we have seen the potential of the products using CrossGlide technology to improve upon current methods. We look forward to bringing the ETS devices to more physicians' offices in the coming months," added Vidyarthi.
CrossBay Medical Inc. was founded in 2009. The company's focus is to change the uterine access experience for both the physician and the patient during office-based procedures.
In today's typical practice, medical providers need to grasp and manipulate the uterine cervix to pass a device for endometrial sampling. This often results in significant discomfort for an awake patient even with pain medication and local anesthetics. The ETS, due to the CrossGlide technology, is designed to require no grasping or manipulation of the cervix to allow for easy, dependable access into the uterine cavity regardless of the complexity or variability of the patient's specific anatomy.
There are approximately 4 million endometrial biopsies performed per year in the European Union and United States for gynecological indications, and there are an additional 2 million endometrial biopsies performed globally for infertility reasons.
"Uterine access in the office doesn't need to be difficult, nor a limit to performing procedures successfully," said Piush Vidyarthi, CEO, CrossBay Medical Inc. "The ETS using the CrossGlide technology was developed with the intent to address the typical discomfort associated with biopsy performed in the diagnostic work-up of abnormal uterine bleeding disorders and infertility. None of the procedures performed with the device required manipulation of the uterus."
Upon receipt of the CE Mark, the ETS using CrossGlide was evaluated by six doctors in Leuven, Belgium; and Naples, Italy.
"We have used the ETS on various patients and can confirm that the passage through the cervix was absolutely easy and the patient felt no pain. Endometrial sampling can be performed under ultrasound visualization and the probe remains in place until the endometrium is removed using a controlled negative pressure," said Dr. Rudi Campo from the Life Expert Centre in Leuven, Belgium.
"The ETS makes crossing the cervical canal much easier because you are not pushing anything across the cervix. Also, since the technology utilizes saline to function, the biopsy can be performed using ultrasound concurrently," said Dr. Attilio Di Spiezio Sardo from University Federico II Hospital in Naples, Italy.
The mission of CrossBay Medical is to empower physicians to deliver compassionate in-office care by offering a suite of products based on the CrossGlide technology. The company will continue to develop and commercialize medical devices using CrossGlide across a wide array of gynecological applications in the coming years.
"At CrossBay Medical, we are using the power of technology to develop products that give physicians the opportunity to bring more procedures into the office. Through our patient evaluations, we have seen the potential of the products using CrossGlide technology to improve upon current methods. We look forward to bringing the ETS devices to more physicians' offices in the coming months," added Vidyarthi.
CrossBay Medical Inc. was founded in 2009. The company's focus is to change the uterine access experience for both the physician and the patient during office-based procedures.