Nova Leah09.25.19
Nova Leah, a cybersecurity solutions provider for medical device manufacturers, announced this week that it has achieved certification to ISO 9001 in recognition of its commitment to quality management systems and ISO 27001 for information security management. The certifications were awarded by the National Standards Authority Ireland (NSAI).
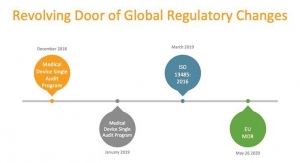
ISO 27001 is the international standard which outlines best practice for implementing the legal, physical and technical controls to ensure confidentiality, integrity and availability of information assets. The ISO 9001 structure provides communication and understanding of the activities that need to take place to produce consistent, high quality product and experience for the customer. Given the company’s overall focus on regulation and compliance, these certifications are both important milestones for the business, especially as it grows its international customer reach.
Nova Leah was founded in 2015 by Dr. Anita Finnegan, government advisor and award-winning international expert in cybersecurity risk management. Its flagship product, SelectEvidence is an expert cybersecurity risk assessment platform that guides medical device manufacturers through the processes of identifying applicable threats to their products and implementing the right security controls to mitigate them. Finnegan is a published author and project leader of multiple, internationally regulated, industry standards for medical equipment in healthcare technology.
She had this to say, “Certification to ISO 9001 and ISO 27001 has resulted in the development of a truly integrated suite of processes that allow us, as an organisation to work in a mode of continuous improvement, with a strong focus on customer satisfaction while ensuring the confidentiality, integrity and availability of all company assets including our products,” said Finnegan.
She added, “Our customers work to very high standards in terms of quality and information security. As a provider of expert cybersecurity risk management solutions, we felt it absolutely necessary that we provide our customers with assurance of our value, appreciation and dedication to high quality standards in terms of products and services.”
The process of becoming ISO certified is extensive, and this involved all departments working together under the guidance of Catherine McEnroe, Nova Leah’s quality manager. McEnroe has had extensive experience in QA and quality engineering.
“The focus of continuous improvement is at the heart of what we do at Nova Leah. ISO certification enables us to build a solid foundation and the means to deliver quality experiences for our customers well into the future,” explained McEnroe.
ISO 9001 and ISO27001 are both published by the International Standardization Organization (ISO) and are globally recognized for the qualification of companies' quality management processes.
For more information, visit http://NovaLeah.com.
ISO 27001 is the international standard which outlines best practice for implementing the legal, physical and technical controls to ensure confidentiality, integrity and availability of information assets. The ISO 9001 structure provides communication and understanding of the activities that need to take place to produce consistent, high quality product and experience for the customer. Given the company’s overall focus on regulation and compliance, these certifications are both important milestones for the business, especially as it grows its international customer reach.
Nova Leah was founded in 2015 by Dr. Anita Finnegan, government advisor and award-winning international expert in cybersecurity risk management. Its flagship product, SelectEvidence is an expert cybersecurity risk assessment platform that guides medical device manufacturers through the processes of identifying applicable threats to their products and implementing the right security controls to mitigate them. Finnegan is a published author and project leader of multiple, internationally regulated, industry standards for medical equipment in healthcare technology.
She had this to say, “Certification to ISO 9001 and ISO 27001 has resulted in the development of a truly integrated suite of processes that allow us, as an organisation to work in a mode of continuous improvement, with a strong focus on customer satisfaction while ensuring the confidentiality, integrity and availability of all company assets including our products,” said Finnegan.
She added, “Our customers work to very high standards in terms of quality and information security. As a provider of expert cybersecurity risk management solutions, we felt it absolutely necessary that we provide our customers with assurance of our value, appreciation and dedication to high quality standards in terms of products and services.”
The process of becoming ISO certified is extensive, and this involved all departments working together under the guidance of Catherine McEnroe, Nova Leah’s quality manager. McEnroe has had extensive experience in QA and quality engineering.
“The focus of continuous improvement is at the heart of what we do at Nova Leah. ISO certification enables us to build a solid foundation and the means to deliver quality experiences for our customers well into the future,” explained McEnroe.
ISO 9001 and ISO27001 are both published by the International Standardization Organization (ISO) and are globally recognized for the qualification of companies' quality management processes.
For more information, visit http://NovaLeah.com.