Business Wire08.28.19
Neurolief, a developer of next-generation digital therapeutics brain neuromodulation technology, has received the CE Mark for its Relivion non-invasive, adaptive digital treatment for migraine. The CE mark allows Neurolief to market, sell and distribute the Relivion device as an over-the-counter therapy within the European Union and countries that participate with agreements on Mutual Recognition of Conformity Assessment.
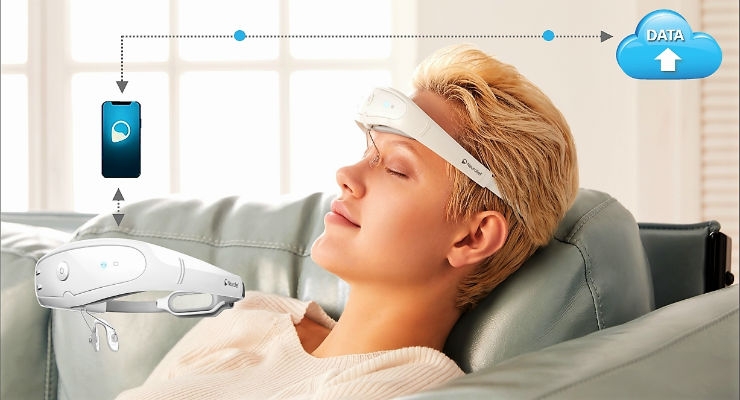
The Relivion is the first non-invasive, adaptive multi-channel brain neuromodulation technology that offers a highly effective therapy, without the risks and costs associated with invasive procedures and without the side effects related to medications. This type of therapy was previously possible only with implanted devices. The Relivion system is simple and safe for patients to self-administer at home at a fraction of the cost of surgical implants.
Neurolief’s Relivion is comprised of a comfortable and adjustable headset that provides precise modulated pulses simultaneously to six branches of the occipital and trigeminal nerves via several adaptive output channels around the patient’s head. The sophisticated cloud-enabled device connects to a proprietary mobile phone app and learns over time to deliver personalized treatment for each patient.
"I believe that the Relivion device from Neurolief has great potential to improve acute migraine therapy," said Alan Rapoport, M.D., clinical professor of neurology at The David Geffen School of Medicine at UCLA (Los Angeles), past president of the International Headache Society (IHS), and the founder and director-emeritus of The New England Center for Headache, in Stamford, Conn. "Not only is it designed to stimulate both the trigeminal and occipital nerves simultaneously to help alleviate migraine without having to worry about side effects from medications, but it does so via a comfortable headset that is different from anything available today. These attributes combine to offer a therapy that migraine sufferers can feel good about using and in turn receive consistent treatment to help them live a more disability-free, productive, fulfilling life."
“This CE mark for the Relivion system marks a vital step in providing patients with alternative, non-drug technology-driven treatments,” said Shmuel Shany, co-founder and CEO, Neurolief. “We designed the Relivion to be a self-administered, safe and attainable alternative to high-cost and high-risk surgical implants, accelerating migraine relief for migraine sufferers in a non-invasive manner. We believe the Relivion will be instrumental in giving patients more rapid relief, increased productivity, and the ability to get back to their lives.”
The CE mark was granted based on data from a randomized, double-blind, placebo-controlled clinical trial which showed very high efficacy and safety of the Relivion in treatment of migraine headache. Seventy-six percent of the participants achieved headache relief after only one treatment while no serious adverse effect were observed.
“Clinical data demonstrate that the Relivion’s confined, closed-loop, interchangeable multi-channel neuromodulation results in a better outcome compared to single-nerve neuromodulation,” said Dr. Eran Schenker, Neurolief’s chief medical innovation officer. “We hypothesize that the disruptive technology synergistic neuromodulatory effect elicited by concurrent activation of both the occipital and trigeminal neural pathways contributes to the superior therapeutic results shown.”
Amit Dar, Neurolief’s co-founder and chief technology officer, added, “Future generations of Neurolief’s innovative technology will include embedded AI and machine learning algorithms, which will provide powerful capabilities to analyze the data collected from each user and from multiple users along with accurate migraine prediction. This will integrate in a seamless fashion to enable updates of the closed-loop personalized brain neuromodulation treatment that improves over time, providing a tailored, precise treatment for each patient. Furthermore, Neurolief is advancing toward implementation of its groundbreaking technology for additional neurological and neuropsychiatric disorders with great unmet need such as major depression.”
Migraine is a prevalent and debilitating primary headache disorder that affects approximately 12 percent of the population worldwide. The World Health Organization (WHO) estimates that 324 million people worldwide suffer from migraines. It is the most prevalent pain condition, prompting loss of productivity with an estimated economic annual burden of over 27 billion euros in European Union countries.1 The economic burden of migraine in the United States was estimated at $36 billion in 2016.2 Migraine patients suffer from disabling symptoms that usually consist of a moderate to severe headache lasting four to 72 hours, nausea and/or vomiting, phonophobia (intolerance to noise) and photophobia (intolerance to light). Seventy-five percent have a reduced ability to function during the migraine episodes, and 33 percent require bed rest during their attacks.3 There is currently no cure for migraines, and over-the-counter medications often do not work. Patients with migraine are often refractory to medical treatment, and some of the most effective antimigraine drugs such as Triptans have substantial side effects.4
Neurolief develops proprietary digital therapeutics brain neuromodulation technology to treat neurological and neuropsychiatric disorders such as migraine and depression. The company’s devices either complement or provide an alternative to pharmaceutical therapies, which are often associated with potential short- and long-term adverse effects. Neurolief’s products are groundbreaking electronic headsets designed to concurrently neuromodulate major neural pathways in the head and thereby affect brain regions that are involved in control and modulation of pain and mood. The devices are intended to provide a highly effective, safe and side effect-free treatment for the hundreds of millions of patients worldwide. Future indications for Neurolief’s technology include depression, insomnia, ADHD and additional chronic pain and neuropsychiatric disorders.
References
1 Stovener, Lars J, and Andrée, Colette, Impact of headache in Europe: A review for the Eurolight project. The Journal of Headache and Pain, 2008. https://www.researchgate.net/publication/5433227
2 Bonafede, M., et al., Study Summary: Costs Associated With Migraine in the United States. AJCM, 2018. https://www.ajmc.com/compendium/migraine/study-summary-costs-associated-with-migraine-in-the-united-states
3 Lipton, R.B., et al., Migraine prevalence, disease burden, and the need for preventive therapy. Neurology, 2007. 68(5): p. 343-9.
4 Loder, E., Triptan therapy in migraine. New England Journal of Medicine, 2010. 363(1): p. 63-70.
The Relivion is the first non-invasive, adaptive multi-channel brain neuromodulation technology that offers a highly effective therapy, without the risks and costs associated with invasive procedures and without the side effects related to medications. This type of therapy was previously possible only with implanted devices. The Relivion system is simple and safe for patients to self-administer at home at a fraction of the cost of surgical implants.
Neurolief’s Relivion is comprised of a comfortable and adjustable headset that provides precise modulated pulses simultaneously to six branches of the occipital and trigeminal nerves via several adaptive output channels around the patient’s head. The sophisticated cloud-enabled device connects to a proprietary mobile phone app and learns over time to deliver personalized treatment for each patient.
"I believe that the Relivion device from Neurolief has great potential to improve acute migraine therapy," said Alan Rapoport, M.D., clinical professor of neurology at The David Geffen School of Medicine at UCLA (Los Angeles), past president of the International Headache Society (IHS), and the founder and director-emeritus of The New England Center for Headache, in Stamford, Conn. "Not only is it designed to stimulate both the trigeminal and occipital nerves simultaneously to help alleviate migraine without having to worry about side effects from medications, but it does so via a comfortable headset that is different from anything available today. These attributes combine to offer a therapy that migraine sufferers can feel good about using and in turn receive consistent treatment to help them live a more disability-free, productive, fulfilling life."
“This CE mark for the Relivion system marks a vital step in providing patients with alternative, non-drug technology-driven treatments,” said Shmuel Shany, co-founder and CEO, Neurolief. “We designed the Relivion to be a self-administered, safe and attainable alternative to high-cost and high-risk surgical implants, accelerating migraine relief for migraine sufferers in a non-invasive manner. We believe the Relivion will be instrumental in giving patients more rapid relief, increased productivity, and the ability to get back to their lives.”
The CE mark was granted based on data from a randomized, double-blind, placebo-controlled clinical trial which showed very high efficacy and safety of the Relivion in treatment of migraine headache. Seventy-six percent of the participants achieved headache relief after only one treatment while no serious adverse effect were observed.
“Clinical data demonstrate that the Relivion’s confined, closed-loop, interchangeable multi-channel neuromodulation results in a better outcome compared to single-nerve neuromodulation,” said Dr. Eran Schenker, Neurolief’s chief medical innovation officer. “We hypothesize that the disruptive technology synergistic neuromodulatory effect elicited by concurrent activation of both the occipital and trigeminal neural pathways contributes to the superior therapeutic results shown.”
Amit Dar, Neurolief’s co-founder and chief technology officer, added, “Future generations of Neurolief’s innovative technology will include embedded AI and machine learning algorithms, which will provide powerful capabilities to analyze the data collected from each user and from multiple users along with accurate migraine prediction. This will integrate in a seamless fashion to enable updates of the closed-loop personalized brain neuromodulation treatment that improves over time, providing a tailored, precise treatment for each patient. Furthermore, Neurolief is advancing toward implementation of its groundbreaking technology for additional neurological and neuropsychiatric disorders with great unmet need such as major depression.”
Migraine is a prevalent and debilitating primary headache disorder that affects approximately 12 percent of the population worldwide. The World Health Organization (WHO) estimates that 324 million people worldwide suffer from migraines. It is the most prevalent pain condition, prompting loss of productivity with an estimated economic annual burden of over 27 billion euros in European Union countries.1 The economic burden of migraine in the United States was estimated at $36 billion in 2016.2 Migraine patients suffer from disabling symptoms that usually consist of a moderate to severe headache lasting four to 72 hours, nausea and/or vomiting, phonophobia (intolerance to noise) and photophobia (intolerance to light). Seventy-five percent have a reduced ability to function during the migraine episodes, and 33 percent require bed rest during their attacks.3 There is currently no cure for migraines, and over-the-counter medications often do not work. Patients with migraine are often refractory to medical treatment, and some of the most effective antimigraine drugs such as Triptans have substantial side effects.4
Neurolief develops proprietary digital therapeutics brain neuromodulation technology to treat neurological and neuropsychiatric disorders such as migraine and depression. The company’s devices either complement or provide an alternative to pharmaceutical therapies, which are often associated with potential short- and long-term adverse effects. Neurolief’s products are groundbreaking electronic headsets designed to concurrently neuromodulate major neural pathways in the head and thereby affect brain regions that are involved in control and modulation of pain and mood. The devices are intended to provide a highly effective, safe and side effect-free treatment for the hundreds of millions of patients worldwide. Future indications for Neurolief’s technology include depression, insomnia, ADHD and additional chronic pain and neuropsychiatric disorders.
References
1 Stovener, Lars J, and Andrée, Colette, Impact of headache in Europe: A review for the Eurolight project. The Journal of Headache and Pain, 2008. https://www.researchgate.net/publication/5433227
2 Bonafede, M., et al., Study Summary: Costs Associated With Migraine in the United States. AJCM, 2018. https://www.ajmc.com/compendium/migraine/study-summary-costs-associated-with-migraine-in-the-united-states
3 Lipton, R.B., et al., Migraine prevalence, disease burden, and the need for preventive therapy. Neurology, 2007. 68(5): p. 343-9.
4 Loder, E., Triptan therapy in migraine. New England Journal of Medicine, 2010. 363(1): p. 63-70.