PR Newswire08.22.19
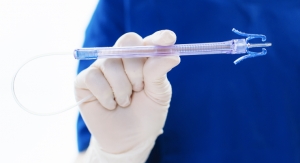
Drawbridge Health today announced that it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for the OneDraw A1C Test System, comprised of the OneDraw Blood Collection Device and the OneDraw A1C Test. The OneDraw A1C Test System is intended for the collection and stabilization of blood and measurement of HbA1c levels for monitoring the long-term control of blood sugar (glucose) in people with diabetes. The OneDraw A1C Test System enables blood to be drawn quickly, comfortably, and conveniently by a health care professional (HCP).
The OneDraw Blood Collection Device is a small, single-use device that draws, collects, and stabilizes a capillary blood sample from the upper arm. Instead of using a traditional hypodermic needle to puncture a patient's vein, the device is placed on the skin and blood is gently collected using tiny lancets with a light vacuum suction. The blood sample is then stabilized and contained within a removable cartridge, designed to protect the sample during transport. After the use of the OneDraw Blood Collection Device, the OneDraw A1C Test is performed by a designated certified clinical laboratory.
The OneDraw Blood Collection Device optimizes the blood collection process for both the patient and HCP and enables blood sampling to be performed anywhere. Drawbridge Health has balanced thoughtful design and proprietary technology to offer patients convenience, comfort, and peace of mind, particularly for patients suffering from the fear of needles. "Patients hate being stuck with needles and many will avoid important testing at all costs. Based on my experience, I believe my patients will be much more comfortable and relaxed—minimal pain and they can't see the blood," comments Kristin Castorino, D.O., Sansum Diabetes Research Institute. Additionally, HCPs will benefit by streamlining the collection process, and ensuring patients complete their necessary blood work with results they can trust.
Drawbridge Health's 510(k) clearance follows the presentation of positive clinical data1 where study results indicated a strong correlation in the HbA1c measurement obtained from samples collected using the OneDraw Blood Collection Device and those using venipuncture. Moreover, the data showed a marked patient preference for the OneDraw Blood Collection device compared to venipuncture or fingerstick.
"Drawbridge Health was founded with the vision and intent to categorically and positively change the nature of blood-based diagnostic testing, improving both the collection process and patient experience," said Lee McCracken, CEO at Drawbridge Health. "Our 510(k) clearance is a critical milestone as we make our founding vision a reality. We look forward to making our novel technology commercially available soon."
Reference
1 Data presented at the 2019 ADA annual conference
The OneDraw Blood Collection Device is a small, single-use device that draws, collects, and stabilizes a capillary blood sample from the upper arm. Instead of using a traditional hypodermic needle to puncture a patient's vein, the device is placed on the skin and blood is gently collected using tiny lancets with a light vacuum suction. The blood sample is then stabilized and contained within a removable cartridge, designed to protect the sample during transport. After the use of the OneDraw Blood Collection Device, the OneDraw A1C Test is performed by a designated certified clinical laboratory.
The OneDraw Blood Collection Device optimizes the blood collection process for both the patient and HCP and enables blood sampling to be performed anywhere. Drawbridge Health has balanced thoughtful design and proprietary technology to offer patients convenience, comfort, and peace of mind, particularly for patients suffering from the fear of needles. "Patients hate being stuck with needles and many will avoid important testing at all costs. Based on my experience, I believe my patients will be much more comfortable and relaxed—minimal pain and they can't see the blood," comments Kristin Castorino, D.O., Sansum Diabetes Research Institute. Additionally, HCPs will benefit by streamlining the collection process, and ensuring patients complete their necessary blood work with results they can trust.
Drawbridge Health's 510(k) clearance follows the presentation of positive clinical data1 where study results indicated a strong correlation in the HbA1c measurement obtained from samples collected using the OneDraw Blood Collection Device and those using venipuncture. Moreover, the data showed a marked patient preference for the OneDraw Blood Collection device compared to venipuncture or fingerstick.
"Drawbridge Health was founded with the vision and intent to categorically and positively change the nature of blood-based diagnostic testing, improving both the collection process and patient experience," said Lee McCracken, CEO at Drawbridge Health. "Our 510(k) clearance is a critical milestone as we make our founding vision a reality. We look forward to making our novel technology commercially available soon."
Reference
1 Data presented at the 2019 ADA annual conference