PR Newswire06.18.19
gel-e Inc. announces Breakthrough Status Designation from the U.S. Food and Drug Administration (FDA) for its first internal-use flowable device.
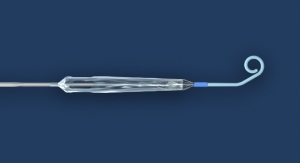
gel-e is developing a line of advanced hemostatic and wound treatment products that address unmet needs from the operating room to the backyard. Following previous clearances for topical and external use of its platform technology, the company is now expanding its label to include use for internal and surgical applications. The first product, now on an accelerated path as a Breakthrough Device, is an expanding injectable hemostat, Life Foam. Life Foam can rapidly provide temporary control of bleeding from non-compressible abdominal wounds that are not amenable to tourniquet application in trauma and battlefield conditions. As the tradename suggests, this product is designed to save the lives of those injured in battle, or that are the victims of traumatic accidents or even terrorist attacks.
Trauma from accidents is the third leading cause of death in the US after only heart disease and cancer. Approximately 85 percent of all hemorrhage-related deaths result from non-compressible internal bleeding, a condition that results from intracavitary (e.g. abdominal) bleeding that is not accessible to direct pressure and cannot be controlled by traditional methods, such as gauze or a tourniquet. Life Foam is designed to dramatically expand once it is delivered into the abdominal cavity where it can seek out the cause of bleeding to rapidly and reliably stabilize the patient or wounded warrior. Once complete, this allows the injured safe passage to a trained trauma surgeon who can repair the damage in a controlled surgical environment, reducing the risks of loss of life or limb. Life Foam is currently in pre-clinical development for the treatment of patients with injuries sustained in battlefield conditions or where life-threatening internal bleeding occurs away from immediate urgent care.
To qualify as a Breakthrough product, a medical device must provide more effective treatment of a life-threatening or irreversibly debilitating condition, and (i) have no approved or cleared alternatives, or (ii) offer significant advantages over existing alternatives, or (iii) be in the best interest of patients. gel-e provided the Agency with information demonstrating that Life Foam meets all these criteria. The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to important breakthrough medical devices by accelerating their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency's mission to protect and promote public health.
"Receiving Breakthrough Device Designation is an important milestone in accelerating the development and clinical implementation of Life Foam, which holds so much potential to save lives on the battlefield and in treating traumatic injuries.," said Dr. Matthew Dowling, Chief Scientific Officer of gel-e. "We look forward to collaborating with the FDA to hasten the clinical development of Life Foam so that it can be used to treat a condition which is otherwise is often deadly or disabling."
Life Foam is just one of the products gel-e plans to develop for internal and surgical applications, all of which will be based on a technology platform that enhances the adhesive characteristics of abundant and inexpensive natural biopolymers. Through proprietary modifications, these advanced materials can be designed to be durable, biocompatible, bioresorbable and highly functional. The versatility of gel-e's approach allows the development and commercialization of easy-to-use products for internal application, including both open and minimally invasive (e.g. laparoscopic) surgical procedures.
gel-e is developing a line of advanced hemostatic and wound treatment products that address unmet needs from the operating room to the backyard. Following previous clearances for topical and external use of its platform technology, the company is now expanding its label to include use for internal and surgical applications. The first product, now on an accelerated path as a Breakthrough Device, is an expanding injectable hemostat, Life Foam. Life Foam can rapidly provide temporary control of bleeding from non-compressible abdominal wounds that are not amenable to tourniquet application in trauma and battlefield conditions. As the tradename suggests, this product is designed to save the lives of those injured in battle, or that are the victims of traumatic accidents or even terrorist attacks.
Trauma from accidents is the third leading cause of death in the US after only heart disease and cancer. Approximately 85 percent of all hemorrhage-related deaths result from non-compressible internal bleeding, a condition that results from intracavitary (e.g. abdominal) bleeding that is not accessible to direct pressure and cannot be controlled by traditional methods, such as gauze or a tourniquet. Life Foam is designed to dramatically expand once it is delivered into the abdominal cavity where it can seek out the cause of bleeding to rapidly and reliably stabilize the patient or wounded warrior. Once complete, this allows the injured safe passage to a trained trauma surgeon who can repair the damage in a controlled surgical environment, reducing the risks of loss of life or limb. Life Foam is currently in pre-clinical development for the treatment of patients with injuries sustained in battlefield conditions or where life-threatening internal bleeding occurs away from immediate urgent care.
To qualify as a Breakthrough product, a medical device must provide more effective treatment of a life-threatening or irreversibly debilitating condition, and (i) have no approved or cleared alternatives, or (ii) offer significant advantages over existing alternatives, or (iii) be in the best interest of patients. gel-e provided the Agency with information demonstrating that Life Foam meets all these criteria. The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to important breakthrough medical devices by accelerating their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency's mission to protect and promote public health.
"Receiving Breakthrough Device Designation is an important milestone in accelerating the development and clinical implementation of Life Foam, which holds so much potential to save lives on the battlefield and in treating traumatic injuries.," said Dr. Matthew Dowling, Chief Scientific Officer of gel-e. "We look forward to collaborating with the FDA to hasten the clinical development of Life Foam so that it can be used to treat a condition which is otherwise is often deadly or disabling."
Life Foam is just one of the products gel-e plans to develop for internal and surgical applications, all of which will be based on a technology platform that enhances the adhesive characteristics of abundant and inexpensive natural biopolymers. Through proprietary modifications, these advanced materials can be designed to be durable, biocompatible, bioresorbable and highly functional. The versatility of gel-e's approach allows the development and commercialization of easy-to-use products for internal application, including both open and minimally invasive (e.g. laparoscopic) surgical procedures.