Business Wire04.24.19
Current Health, an artificial intelligence (AI)-powered wearable remote patient monitoring platform (RPM), has received Class II clearance from the U.S. Food and Drug Administration (FDA) for post-acute care. Available for both hospital and home, Current has achieved the first-ever FDA clearance for an end-to-end, real-time, passive RPM wearable and platform.
The post-acute FDA clearance comes as Current is experiencing overwhelming customer demand for its platform, combining its all-in-one wireless wearable, which provides ICU-level accuracy and tracks more vital signs than any other all-in-one wearable available today, with analytics to derive actionable insights. Current has already partnered with six of the largest health systems in the U.S., including Mount Sinai, and with several U.K. NHS Trusts, assisting them in delivering healthcare at home earlier to reduce readmissions and preventable deaths.
As the healthcare industry shifts to value-based care, providers must intervene earlier to improve patient outcomes, decrease lengths of stay and reduce hospital readmissions. However, providers have no means of identifying at-risk patients after they leave the hospital. Traditional RPM and telehealth solutions cannot meet these challenges due to continued reliance on manual data entry, unreliable spot measurements, a lack of widespread adoption and low patient adherence.
"Real-time, at-home monitoring of vitals allows our team to proactively act on early signs of health decline, preventing avoidable hospitalizations," said Dr. Neta Faynboym M.D., CPE Executive Director, Innovation: Medicare Advantage/Affordability, at Banner Health.
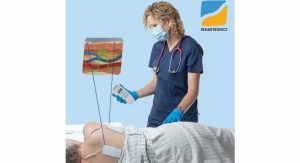
Designed specifically with the patient in mind, Current passively measures a patient’s vital signs in real time with its upper-arm wearable. Developed using one of the world’s largest physiological data sets, Current’s proprietary algorithms continuously analyze data to derive a patient’s health trajectory by detecting potential indicators of patient decline earlier for faster intervention.
Current received FDA clearance for its entire RPM and telehealth platform, which includes Bluetooth integrations with other best-in-class devices to track metrics. Patients receive a tablet equipped with a chatbot for Q&A, medication reminders and educational content, which lets patients connect with clinicians via video chat or text message to report symptoms and discuss care.
“Our rapidly growing customer base indicates how focused health systems and home health agencies are on moving more healthcare from hospital to home,” said Current Health CEO and co-founder Christopher McCann. “Today, Current is helping them do just that by monitoring patients’ health trajectories to enable earlier interventions, reduce the overall and growing cost of hospital readmissions and, more importantly, prevent avoidable deaths. But more fundamentally, we’re building a future where healthcare comes to us. Patients don’t always know when to call their doctor. Current will.”
The post-acute FDA clearance comes as Current is experiencing overwhelming customer demand for its platform, combining its all-in-one wireless wearable, which provides ICU-level accuracy and tracks more vital signs than any other all-in-one wearable available today, with analytics to derive actionable insights. Current has already partnered with six of the largest health systems in the U.S., including Mount Sinai, and with several U.K. NHS Trusts, assisting them in delivering healthcare at home earlier to reduce readmissions and preventable deaths.
As the healthcare industry shifts to value-based care, providers must intervene earlier to improve patient outcomes, decrease lengths of stay and reduce hospital readmissions. However, providers have no means of identifying at-risk patients after they leave the hospital. Traditional RPM and telehealth solutions cannot meet these challenges due to continued reliance on manual data entry, unreliable spot measurements, a lack of widespread adoption and low patient adherence.
"Real-time, at-home monitoring of vitals allows our team to proactively act on early signs of health decline, preventing avoidable hospitalizations," said Dr. Neta Faynboym M.D., CPE Executive Director, Innovation: Medicare Advantage/Affordability, at Banner Health.
Designed specifically with the patient in mind, Current passively measures a patient’s vital signs in real time with its upper-arm wearable. Developed using one of the world’s largest physiological data sets, Current’s proprietary algorithms continuously analyze data to derive a patient’s health trajectory by detecting potential indicators of patient decline earlier for faster intervention.
Current received FDA clearance for its entire RPM and telehealth platform, which includes Bluetooth integrations with other best-in-class devices to track metrics. Patients receive a tablet equipped with a chatbot for Q&A, medication reminders and educational content, which lets patients connect with clinicians via video chat or text message to report symptoms and discuss care.
“Our rapidly growing customer base indicates how focused health systems and home health agencies are on moving more healthcare from hospital to home,” said Current Health CEO and co-founder Christopher McCann. “Today, Current is helping them do just that by monitoring patients’ health trajectories to enable earlier interventions, reduce the overall and growing cost of hospital readmissions and, more importantly, prevent avoidable deaths. But more fundamentally, we’re building a future where healthcare comes to us. Patients don’t always know when to call their doctor. Current will.”