PR Newswire04.24.19
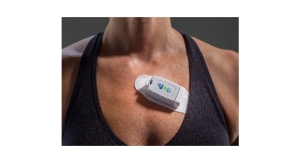
AliveCor, the developer of FDA-cleared consumer electrocardiogram technology (ECG), announced two additional FDA 510(k)-cleared indications, making KardiaMobile the only consumer ECG device in the world with FDA-clearance to detect the three most common heart arrhythmias.
In addition to detecting Atrial Fibrillation (AFib) and Normal Sinus Rhythm, AliveCor is able to detect and show ECG results for Bradycardia and Tachycardia. These instant analyses indicate arrhythmias that are not AFib and that are between 40-50 beats per minute (Bradycardia), or between 100-140 beats per minute (Tachycardia).
"No other consumer ECG device in the world, can tell you more about your heart than KardiaMobile," said AliveCor CEO, Ira Bahr. "Until today, patients have been frustrated when devices label their ECG reading as 'unclassified' or 'inconclusive.' Starting today, KardiaMobile is the first personal ECG device that can begin to materially reduce the number of those determinations. Critically, KardiaMobile is also the only personal ECG that can detect Atrial Fibrillation at heart rates above 120, and heart rates below 40."
Bradycardia and tachycardia, which literally mean "slow heart" and "fast heart", respectively, occur in nearly all adults. While the normal range of heart rate is between 50 and 100 beats per minute, there are many scenarios where the rate can be slightly out of this range. For example, the heart may slow below 50 beats per minute during sleep, and naturally, in many healthy adults and athletes. A fast heart rate may naturally occur in response to exercise, anxiety, or pain.
While Bradycardia and Tachycardia are often benign, these arrhythmias can be indicative of heart disease or other health conditions, such as thyroid disease. A slow or fast heart rate may be asymptomatic, or cause symptoms such as dizziness or shortness of breath. KardiaMobile users will now be able to detect these arrhythmias and use the insight to inform conversations with their doctor. Beyond the patient-doctor relationship, KardiaMobile also provides peace of mind by diminishing the number of unclassified readings that users may receive.
"Tachycardia and bradycardia are common because they are often the body's natural response to everyday life, from physical activity and sleep to emotions and overall health," said Dr. Jacqueline Shreibati, CMO of AliveCor. "While we have traditionally focused on the patient empowerment that comes from increased awareness of atrial fibrillation, we are excited to give all of our users more actionable insights into their heart health."
In addition to detecting Atrial Fibrillation (AFib) and Normal Sinus Rhythm, AliveCor is able to detect and show ECG results for Bradycardia and Tachycardia. These instant analyses indicate arrhythmias that are not AFib and that are between 40-50 beats per minute (Bradycardia), or between 100-140 beats per minute (Tachycardia).
"No other consumer ECG device in the world, can tell you more about your heart than KardiaMobile," said AliveCor CEO, Ira Bahr. "Until today, patients have been frustrated when devices label their ECG reading as 'unclassified' or 'inconclusive.' Starting today, KardiaMobile is the first personal ECG device that can begin to materially reduce the number of those determinations. Critically, KardiaMobile is also the only personal ECG that can detect Atrial Fibrillation at heart rates above 120, and heart rates below 40."
Bradycardia and tachycardia, which literally mean "slow heart" and "fast heart", respectively, occur in nearly all adults. While the normal range of heart rate is between 50 and 100 beats per minute, there are many scenarios where the rate can be slightly out of this range. For example, the heart may slow below 50 beats per minute during sleep, and naturally, in many healthy adults and athletes. A fast heart rate may naturally occur in response to exercise, anxiety, or pain.
While Bradycardia and Tachycardia are often benign, these arrhythmias can be indicative of heart disease or other health conditions, such as thyroid disease. A slow or fast heart rate may be asymptomatic, or cause symptoms such as dizziness or shortness of breath. KardiaMobile users will now be able to detect these arrhythmias and use the insight to inform conversations with their doctor. Beyond the patient-doctor relationship, KardiaMobile also provides peace of mind by diminishing the number of unclassified readings that users may receive.
"Tachycardia and bradycardia are common because they are often the body's natural response to everyday life, from physical activity and sleep to emotions and overall health," said Dr. Jacqueline Shreibati, CMO of AliveCor. "While we have traditionally focused on the patient empowerment that comes from increased awareness of atrial fibrillation, we are excited to give all of our users more actionable insights into their heart health."