Globe Newswire04.12.19
PAVmed Inc. subsidiary Lucid Diagnostics Inc. (Lucid), has launched a medical advisory board (MAB) consisting of internationally renowned experts in gastroesophageal reflux disease (GERD), Barrett’s Esophagus (BE) and esophageal cancer and has appointed veteran life sciences industry executive David Wurtman, M.D., MBA, as its chief medical officer.
The Lucid Medical Advisory Board members include:
Lucid MAB members have led the development of published guidelines on the management of Barrett’s Esophagus sanctioned by both major gastroenterology societies. Drs. Shaheen and Falk are lead authors of the current American College of Gastroenterology (ACG) guidelines on the Diagnosis and Management of Barrett’s Esophagus. Drs. Sharma and Shaheen are authors of the current American Gastroenterological Association Technical Review on the Management of Barrett's Esophagus.
Lucid MAB members are co-founders, investigators and participants of all of the major clinical and research initiatives in the field of Barrett’s Esophagus including the National Cancer Institute’s (NCI) Barrett’s Esophagus Translational Research Network (BETRNet), GI Specialized Program of Research Excellence (GI SPORE) Centers, the Early Detection Research Network (EDRN), Barrett’s Esophageal and Adenocarcinoma Consortium (BEACON), and the Familial Barrett’s Esophagus Consortium (FBEC). Dr. Chak is an EsoCheck co-inventor and principal investigator of the ongoing National Institutes of Health-funded clinical trial of EsoCheck which includes Dr. Shaheen and his team.
Lucid MAB members also serve on the editorial boards of the major gastroenterology journals including Gastroenterology, the American Journal of Gastroenterology, Clinical Gastroenterology and Hepatology, Gastrointestinal Endoscopy, and the Journal of Clinical Gastroenterology. They have collectively published hundreds of peer reviewed journal articles and served as lead editor and co-author of the definitive textbook, "Barrett’s Esophagus and Esophageal Adenocarcinoma."
“I am honored to join my esteemed colleagues on Lucid Diagnostics’ Medical Advisory Board and to serve as its chair as it develops and executes its long-term clinical and regulatory plan for its EsoCheck technology,” said Dr. Shaheen. “I have spent my career seeking to improve the care of patients with GERD/Barrett’s Esophagus and to prevent deaths from esophageal cancer. I have actively participated in the development of key advances in this field and am particularly excited about the potential for the EsoCheck technology to save lives through the early detection of Barrett’s Esophagus.”
Wurtman, joins PAVmed as its executive vice president, Strategic Projects and Lucid’s chief medical officer. Dr. Wurtman is a veteran life sciences industry executive who has held leadership roles in corporate development, business development and licensing, marketing, product development, and medical affairs for over two decades.
He most recently served as president, CEO, and co-founder of Lyric Pharmaceuticals, which sought to develop novel therapeutics to improve the care of critically ill and hospitalized patients, including the treatment of enteral feeding intolerance in the intensive care unit. Dr. Wurtman led his team in completing a definitive international multi-center Phase 2 trial of ulimorelin, an IV ghrelin agonist. Prior to co-founding Lyric, he served as vice president of Medical Affairs and Product Development at Kineta Inc., a clinical stage biotechnology company, and maintained a successful practice as an independent corporate and product development consultant. Dr. Wurtman has held senior positions at Protein Design Labs (now PDL Biopharma), Eos Biotechnology Inc., and Genzyme Corporation (now Sanofi Genzyme), and worked in the pharmaceutical analyst group at SG Cowen (now Cowen Inc.). He has executed numerous licensing and partnering transactions and capital raises including a $33.8 million funding round for Lyric.
“Dr. Wurtman brings an enormous amount of executive experience in the development and execution of clinical and regulatory strategy, including sophisticated clinical trials, to his role as Lucid’s chief medical officer,” said Dr. Aklog. “I am excited that he will lead EsoCheck’s clinical and regulatory efforts. He will apply his expertise to the design and execution of Lucid-sponsored clinical studies of EsoCheck, in close collaboration with our MAB and former FDA officials retained through a leading regulatory consulting firm with the ultimate goal of securing a specific indication for widespread Barrett’s Esophagus screening using EsoCheck technology.”
Dr. Wurtman received his AB from Harvard College, his medical degree from Harvard Medical School, and his MBA from the MIT Sloan School of Management. Prior to entering the life sciences industry, he completed a primary care internal medicine residency at Mt. Auburn Hospital, a Harvard teaching hospital, and practiced primary care at Harvard Community Health Plan as a board-certified physician in Internal Medicine.
“I am excited to join the PAVmed team and to serve as chief medical officer of Lucid Diagnostics,” said Dr. Wurtman. “Over the past five months I’ve worked closely with the Lucid team to build the vision and operations for our clinical and regulatory strategy to advance EsoCheck. It is my hope that EsoCheck will benefit the tens of millions of patients with GERD who are at risk for Barrett’s Esophagus and esophageal cancer. This ongoing work, in conjunction with the members of our world-class MAB, positions Lucid well to advance EsoCheck successfully through regulatory clearance and potential commercialization.”
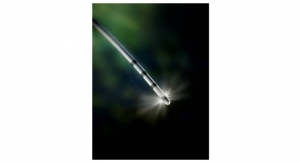
EsoCheck is a technology licensed by PAVmed’s majority-owned subsidiary, Lucid Diagnostics Inc., which was highlighted as one of the year’s significant advances in cancer prevention in the National Cancer Institute’s 2020 Annual Plan and Budget Proposal submitted to Congress. It consists of two distinct products. EsoCheck CCD is a balloon catheter designed to collect cells from a targeted region of the esophagus in a five-minute office-based procedure, without the need for endoscopy. EsoCheck Dx is a methylated DNA biomarker test (mVIM + mCCNA1) which has been shown in a published human study to be highly accurate at detecting Barrett’s Esophagus (BE), a pre-cursor to highly lethal esophageal cancer in patients with chronic heart burn or acid reflux (GERD).
The company believes the EsoCheck technology has the potential to save many lives through early BE detection, with an immediately addressable domestic market opportunity of at least $2 billion based on tens of millions U.S. GERD patients who are BE screening candidates based on published guidelines. Lucid is pursuing a two-phase regulatory and commercialization strategy which seeks to maximize the EsoCheck technology’s long-term commercial opportunity while providing near-term value-inflection commercial milestones. The first phase seeks to commercially launch EsoCheck CCD as a U.S. Food and Drug Administration (FDA) 510(k)-cleared cell collection device and separately launch EsoCheck Dx as a Laboratory Developed Test (LDT), which does not currently require FDA review. The second phase seeks a specific indication for widespread BE screening using the two EsoCheck products based on published guidelines.
PAVmed Inc. is a highly differentiated, multiproduct medical device company employing a unique business model designed to advance products to commercialization much more rapidly and with significantly less capital than the typical medical device company. This proprietary model enables PAVmed to pursue an expanding pipeline strategy with a view to enhancing and accelerating value creation. PAVmed’s pipeline of products address unmet clinical needs encompassing a broad spectrum of clinical areas with attractive regulatory pathways and market opportunities. Its five lead products provide groundbreaking approaches to carpal tunnel syndrome (CarpX), precancerous conditions of the esophagus (EsoCheck), vascular access (PortIO), pediatric ear infections (DisappEAR) and medical infusions (NextFlo). The company is also developing products in other areas, such as catheters and tissue ablation, while seeking to further expand its pipeline through engagements with clinician innovators and leading academic medical centers.
The Lucid Medical Advisory Board members include:
- Chairman Nicholas J. Shaheen, M.D., MPH, a professor of Medicine and Epidemiology at the University of North Carolina School of Medicine and University of North Carolina School of Public Health. He is chief of the Division of Gastroenterology and Hepatology UNC HealthCare, Chapel Hill, N.C. Shaheen also is director of the American College of Gastroentrology Institute for Clinical Research and Education.
- Ambitabh Chak, M.D., a professor of Medicine at Case Western Reserve University School of Medicine. Chak is director of the Advanced Technology & Innovation Center of Excellence at University Hospitals Cleveland Medical Center, Division of Gastroenterology, in Cleveland, Ohio.
- Gary W. Falk, M.D., MS, a professor of Medicine at the University of Pennsylvania Perelman School of Medicine. He also is clinical co-director, Joint Center for Digestive, Liver and Pancreatic Medicine; co-director, Esophagology and Swallowing Center, Hospital of the University of Pennsylvania (Philadelphia); and past president of the American Society of Gastrointestinal Endoscopy.
- Anthony Infantolino, M.D., a professor of Medicine at Thomas Jefferson University Sidney Kimmel Medical College. He also is director, Barrett's Esophagus Treatment Center; director, Endoscopic Ultrasound Unit; and co-director, Jefferson GI Bleeding Center, Thomas Jefferson University Hospital in Philadelphia, Pa.
- Prateek Sharma, M.D., a professor of Medicine at the University of Kansas School of Medicine. He also is director, Gastroenterology Fellowship Program; section chief, Veterans Affairs Medical Center in Kansas City, Mo.; and vice president, The International Society for Diseases of the Esophagus.
- Michael S. Smith, M.D., MBA, an associate professor of Medicine at the Icahn School of Medicine at Mount Sinai. He also is chief of Gastroenterology and Hepatology at Mount Sinai West and Mount Sinai St. Luke's Hospitals in New York, N.Y.
Lucid MAB members have led the development of published guidelines on the management of Barrett’s Esophagus sanctioned by both major gastroenterology societies. Drs. Shaheen and Falk are lead authors of the current American College of Gastroenterology (ACG) guidelines on the Diagnosis and Management of Barrett’s Esophagus. Drs. Sharma and Shaheen are authors of the current American Gastroenterological Association Technical Review on the Management of Barrett's Esophagus.
Lucid MAB members are co-founders, investigators and participants of all of the major clinical and research initiatives in the field of Barrett’s Esophagus including the National Cancer Institute’s (NCI) Barrett’s Esophagus Translational Research Network (BETRNet), GI Specialized Program of Research Excellence (GI SPORE) Centers, the Early Detection Research Network (EDRN), Barrett’s Esophageal and Adenocarcinoma Consortium (BEACON), and the Familial Barrett’s Esophagus Consortium (FBEC). Dr. Chak is an EsoCheck co-inventor and principal investigator of the ongoing National Institutes of Health-funded clinical trial of EsoCheck which includes Dr. Shaheen and his team.
Lucid MAB members also serve on the editorial boards of the major gastroenterology journals including Gastroenterology, the American Journal of Gastroenterology, Clinical Gastroenterology and Hepatology, Gastrointestinal Endoscopy, and the Journal of Clinical Gastroenterology. They have collectively published hundreds of peer reviewed journal articles and served as lead editor and co-author of the definitive textbook, "Barrett’s Esophagus and Esophageal Adenocarcinoma."
“I am honored to join my esteemed colleagues on Lucid Diagnostics’ Medical Advisory Board and to serve as its chair as it develops and executes its long-term clinical and regulatory plan for its EsoCheck technology,” said Dr. Shaheen. “I have spent my career seeking to improve the care of patients with GERD/Barrett’s Esophagus and to prevent deaths from esophageal cancer. I have actively participated in the development of key advances in this field and am particularly excited about the potential for the EsoCheck technology to save lives through the early detection of Barrett’s Esophagus.”
Wurtman, joins PAVmed as its executive vice president, Strategic Projects and Lucid’s chief medical officer. Dr. Wurtman is a veteran life sciences industry executive who has held leadership roles in corporate development, business development and licensing, marketing, product development, and medical affairs for over two decades.
He most recently served as president, CEO, and co-founder of Lyric Pharmaceuticals, which sought to develop novel therapeutics to improve the care of critically ill and hospitalized patients, including the treatment of enteral feeding intolerance in the intensive care unit. Dr. Wurtman led his team in completing a definitive international multi-center Phase 2 trial of ulimorelin, an IV ghrelin agonist. Prior to co-founding Lyric, he served as vice president of Medical Affairs and Product Development at Kineta Inc., a clinical stage biotechnology company, and maintained a successful practice as an independent corporate and product development consultant. Dr. Wurtman has held senior positions at Protein Design Labs (now PDL Biopharma), Eos Biotechnology Inc., and Genzyme Corporation (now Sanofi Genzyme), and worked in the pharmaceutical analyst group at SG Cowen (now Cowen Inc.). He has executed numerous licensing and partnering transactions and capital raises including a $33.8 million funding round for Lyric.
“Dr. Wurtman brings an enormous amount of executive experience in the development and execution of clinical and regulatory strategy, including sophisticated clinical trials, to his role as Lucid’s chief medical officer,” said Dr. Aklog. “I am excited that he will lead EsoCheck’s clinical and regulatory efforts. He will apply his expertise to the design and execution of Lucid-sponsored clinical studies of EsoCheck, in close collaboration with our MAB and former FDA officials retained through a leading regulatory consulting firm with the ultimate goal of securing a specific indication for widespread Barrett’s Esophagus screening using EsoCheck technology.”
Dr. Wurtman received his AB from Harvard College, his medical degree from Harvard Medical School, and his MBA from the MIT Sloan School of Management. Prior to entering the life sciences industry, he completed a primary care internal medicine residency at Mt. Auburn Hospital, a Harvard teaching hospital, and practiced primary care at Harvard Community Health Plan as a board-certified physician in Internal Medicine.
“I am excited to join the PAVmed team and to serve as chief medical officer of Lucid Diagnostics,” said Dr. Wurtman. “Over the past five months I’ve worked closely with the Lucid team to build the vision and operations for our clinical and regulatory strategy to advance EsoCheck. It is my hope that EsoCheck will benefit the tens of millions of patients with GERD who are at risk for Barrett’s Esophagus and esophageal cancer. This ongoing work, in conjunction with the members of our world-class MAB, positions Lucid well to advance EsoCheck successfully through regulatory clearance and potential commercialization.”
EsoCheck is a technology licensed by PAVmed’s majority-owned subsidiary, Lucid Diagnostics Inc., which was highlighted as one of the year’s significant advances in cancer prevention in the National Cancer Institute’s 2020 Annual Plan and Budget Proposal submitted to Congress. It consists of two distinct products. EsoCheck CCD is a balloon catheter designed to collect cells from a targeted region of the esophagus in a five-minute office-based procedure, without the need for endoscopy. EsoCheck Dx is a methylated DNA biomarker test (mVIM + mCCNA1) which has been shown in a published human study to be highly accurate at detecting Barrett’s Esophagus (BE), a pre-cursor to highly lethal esophageal cancer in patients with chronic heart burn or acid reflux (GERD).
The company believes the EsoCheck technology has the potential to save many lives through early BE detection, with an immediately addressable domestic market opportunity of at least $2 billion based on tens of millions U.S. GERD patients who are BE screening candidates based on published guidelines. Lucid is pursuing a two-phase regulatory and commercialization strategy which seeks to maximize the EsoCheck technology’s long-term commercial opportunity while providing near-term value-inflection commercial milestones. The first phase seeks to commercially launch EsoCheck CCD as a U.S. Food and Drug Administration (FDA) 510(k)-cleared cell collection device and separately launch EsoCheck Dx as a Laboratory Developed Test (LDT), which does not currently require FDA review. The second phase seeks a specific indication for widespread BE screening using the two EsoCheck products based on published guidelines.
PAVmed Inc. is a highly differentiated, multiproduct medical device company employing a unique business model designed to advance products to commercialization much more rapidly and with significantly less capital than the typical medical device company. This proprietary model enables PAVmed to pursue an expanding pipeline strategy with a view to enhancing and accelerating value creation. PAVmed’s pipeline of products address unmet clinical needs encompassing a broad spectrum of clinical areas with attractive regulatory pathways and market opportunities. Its five lead products provide groundbreaking approaches to carpal tunnel syndrome (CarpX), precancerous conditions of the esophagus (EsoCheck), vascular access (PortIO), pediatric ear infections (DisappEAR) and medical infusions (NextFlo). The company is also developing products in other areas, such as catheters and tissue ablation, while seeking to further expand its pipeline through engagements with clinician innovators and leading academic medical centers.