Globe Newswire04.03.19
BioSig Technologies Inc., a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the electrophysiology (EP) marketplace, announced that the company successfully conducted first patient cases using PURE EP System, its U.S. Food and Drug Administration (FDA)-approved proprietary signal acquisition and processing technology. The first commercial use of the System was completed at the Texas Cardiac Arrhythmia Institute (TCAI) in Austin, Texas.
The patient studies were conducted by Andrea Natale, M.D., F.A.C.C., F.H.R.S., F.E.S.C., executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center.
“With the use of the PURE EP System I was able to identify cardiac signals which were previously undetectable to me. I believe the PURE EP System could change diagnostic and treatment strategies of arrhythmias, leading to more successful outcomes,” commented Dr. Natale.
The PURE EP System was used during the standard studies on patients with persistent atrial fibrillation and conducted in parallel with Abbott’s EnSite Precision and Biosense Webster’s (Johnson & Johnson) CARTO cardiac mapping systems. The goal of the first commercial use of the technology was aimed at validating the System’s key value proposition elements and report on the overall user experience during the procedure.
“We are highly encouraged by the first results reported by Dr. Natale and his team at this facility. A positive first-in-human experience is a major inflection point for our company and lays a strong foundation for our early commercialization efforts,” commented Kenneth L. Londoner, chairman and CEO of BioSig Technologies. “We are well positioned to deliver on our strategic goals for 2019 and look forward to the expansion of our evaluation efforts in the coming months.”
The company released its shareholder letter in February 2019, where it stated its intentions to present the results from the first-in-human studies and the early feedback from the use of the PURE EP System to a larger community of physicians during the Heart Rhythm Society event in May 2019.
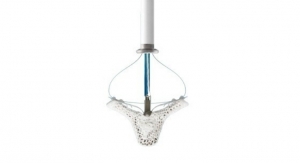
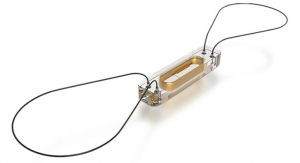
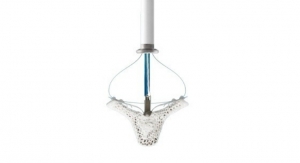
The PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients undergoing electrophysiology (EP) procedures in an EP laboratory. The PURE EP System aims to minimize noise and artifacts and acquire high-fidelity cardiac signals. Improving fidelity of acquired cardiac signals may potentially increase the diagnostic value of these signals, thereby possibly improving accuracy and efficiency of the EP studies and related procedures. The results of pre-clinical studies have been published in a number of journals, including The Journal of Innovations in Cardiac Rhythm Management.
Located at St. David’s Medical Center in Austin, Texas, the Texas Cardiac Arrhythmia Institute is a recognized training, research and treatment facility dedicated solely to heart rhythm disorders. St. David’s is a state-of-the-science medical center with a medical support staff. Its robotics, magnetics and other advanced technologies complement the expertise of TCAI’s physicians. The Institute brings TCAI’s respected physicians and researchers together with St. David’s superior facilities to address even the most challenging arrhythmias.
BioSig Technologies is a medical technology company developing a proprietary biomedical signal processing platform designed to improve the electrophysiology (EP) marketplace. Led by a management team and a veteran Board of Directors, BioSig Technologies is preparing to commercialize its PURE EP System. The technology has been developed to address an unmet need in a large and growing market.
The ompany’s first product, PURE EP System, is a novel cardiac signal acquisition and display system which is engineered to assist electrophysiologists in clinical decision-making during procedures to diagnose and treat patients with abnormal heart rates and rhythms. BioSig’s main goal is to deliver technology to improve upon catheter ablation treatments for the prevalent and potentially deadly arrhythmias, atrial fibrillation and ventricular tachycardia. BioSig has partnered with Minnetronix on technology development and has received FDA 510(k) clearance for the PURE EP System in August 2018.
The patient studies were conducted by Andrea Natale, M.D., F.A.C.C., F.H.R.S., F.E.S.C., executive medical director, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center.
“With the use of the PURE EP System I was able to identify cardiac signals which were previously undetectable to me. I believe the PURE EP System could change diagnostic and treatment strategies of arrhythmias, leading to more successful outcomes,” commented Dr. Natale.
The PURE EP System was used during the standard studies on patients with persistent atrial fibrillation and conducted in parallel with Abbott’s EnSite Precision and Biosense Webster’s (Johnson & Johnson) CARTO cardiac mapping systems. The goal of the first commercial use of the technology was aimed at validating the System’s key value proposition elements and report on the overall user experience during the procedure.
“We are highly encouraged by the first results reported by Dr. Natale and his team at this facility. A positive first-in-human experience is a major inflection point for our company and lays a strong foundation for our early commercialization efforts,” commented Kenneth L. Londoner, chairman and CEO of BioSig Technologies. “We are well positioned to deliver on our strategic goals for 2019 and look forward to the expansion of our evaluation efforts in the coming months.”
The company released its shareholder letter in February 2019, where it stated its intentions to present the results from the first-in-human studies and the early feedback from the use of the PURE EP System to a larger community of physicians during the Heart Rhythm Society event in May 2019.
The PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients undergoing electrophysiology (EP) procedures in an EP laboratory. The PURE EP System aims to minimize noise and artifacts and acquire high-fidelity cardiac signals. Improving fidelity of acquired cardiac signals may potentially increase the diagnostic value of these signals, thereby possibly improving accuracy and efficiency of the EP studies and related procedures. The results of pre-clinical studies have been published in a number of journals, including The Journal of Innovations in Cardiac Rhythm Management.
Located at St. David’s Medical Center in Austin, Texas, the Texas Cardiac Arrhythmia Institute is a recognized training, research and treatment facility dedicated solely to heart rhythm disorders. St. David’s is a state-of-the-science medical center with a medical support staff. Its robotics, magnetics and other advanced technologies complement the expertise of TCAI’s physicians. The Institute brings TCAI’s respected physicians and researchers together with St. David’s superior facilities to address even the most challenging arrhythmias.
BioSig Technologies is a medical technology company developing a proprietary biomedical signal processing platform designed to improve the electrophysiology (EP) marketplace. Led by a management team and a veteran Board of Directors, BioSig Technologies is preparing to commercialize its PURE EP System. The technology has been developed to address an unmet need in a large and growing market.
The ompany’s first product, PURE EP System, is a novel cardiac signal acquisition and display system which is engineered to assist electrophysiologists in clinical decision-making during procedures to diagnose and treat patients with abnormal heart rates and rhythms. BioSig’s main goal is to deliver technology to improve upon catheter ablation treatments for the prevalent and potentially deadly arrhythmias, atrial fibrillation and ventricular tachycardia. BioSig has partnered with Minnetronix on technology development and has received FDA 510(k) clearance for the PURE EP System in August 2018.