Business Wire03.21.19
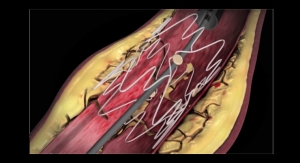
Confluent Medical (formerly Nitinol Devices & Components, “NDC”) has launched the High-Performance Nitinol Tubing Center of Excellence in response to increasing market demand for high-performance nitinol tubing in challenging applications such as transcatheter heart valves, neurovascular, electrophysiology, and interventional pulmonary.
The High-Performance Nitinol Tubing Center of Excellence has internal capabilities that include:
The acquisition of Tube Hollows International has been a key piece in the development of the High-Performance Nitinol Tubing Center of Excellence. THI is the leader in precision gun-drilling of nitinol tube hollows. Additionally, THI brings capabilities in centerless grinding and precision machining.
Confluent Medical’s High-Performance Nitinol Tubing Center of Excellence is ideally set up to support medical device companies that require world-class nitinol tubing for their designs for long-term implantable applications within the heart and other critical structures.
Confluent Medical Technologies is the largest contract manufacturer of specialized medical devices. Its portfolio of services includes nitinol components, biomedical textiles, balloon expandable stents and balloon catheters, delivery systems, access kits, and guidewires. With manufacturing facilities in Fremont, Calif.; Sunnyvale, Calif.; Laguna Niguel, Calif.; Warwick, R.I.; and San Jose, Costa Rica, Confluent Medical Technologies has a 20-year track record of partnering with the medical device community and delivering high-quality medical devices.
The High-Performance Nitinol Tubing Center of Excellence has internal capabilities that include:
- High purity ELI Nitinol material. Custom-melted nitinol material with exceptionally high purity that provides superior cyclical fatigue durability.
- Wide range of nitinol tubing sizes and tolerances. Nitinol tubing in diameters that exceed 10mm, which is often utilized in complex transcatheter mitral or aortic valve components.
- Precise nitinol tubing processes, including gun-drilling, centerless grinding, and machining. With the recent acquisition of Tube Hollows International, Confluent Medical has a best-in-class offering for nitinol tubing processing.
- Superior surface finish. An optimal surface on the OD and ID that allows for ideal component manufacturing.
The acquisition of Tube Hollows International has been a key piece in the development of the High-Performance Nitinol Tubing Center of Excellence. THI is the leader in precision gun-drilling of nitinol tube hollows. Additionally, THI brings capabilities in centerless grinding and precision machining.
Confluent Medical’s High-Performance Nitinol Tubing Center of Excellence is ideally set up to support medical device companies that require world-class nitinol tubing for their designs for long-term implantable applications within the heart and other critical structures.
Confluent Medical Technologies is the largest contract manufacturer of specialized medical devices. Its portfolio of services includes nitinol components, biomedical textiles, balloon expandable stents and balloon catheters, delivery systems, access kits, and guidewires. With manufacturing facilities in Fremont, Calif.; Sunnyvale, Calif.; Laguna Niguel, Calif.; Warwick, R.I.; and San Jose, Costa Rica, Confluent Medical Technologies has a 20-year track record of partnering with the medical device community and delivering high-quality medical devices.