Boston Scientific03.13.19
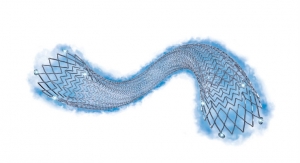
Boston Scientific Corporation announced it has received CE mark and initiated a limited market release of the next generation WATCHMAN FLX Left Atrial Appendage Closure (LAAC) Device in Europe.
Patients with AF are five times more likely to suffer a stroke than someone with a normal heart rhythm.1 In people with non-valvular atrial fibrillation (AF), data suggests that more than 90 percent of stroke-causing blood clots that come from the heart are formed in the left atrial appendage.2 The WATCHMAN left atrial appendage occlusion devices are intended to reduce the risk of stroke in people with non-valvular AF.
"The WATCHMAN device has been implanted in more than 75,000 patients worldwide and we are pleased that this next-generation technology has been granted European regulatory approval so that we can offer it to patients and clinicians throughout Europe," said Kevin Ballinger, president, Interventional Cardiology, Boston Scientific. "The robust clinical evidence and successful commercial outcomes of the WATCHMAN device to-date reinforce the value of this procedure for all appropriate patients."
The new WATCHMAN FLX device was designed for simplified implantation to fit a wider range of patients, from those with simple to the most complex anatomies. The device allows for implantation flexibility to customize placement with a fully enclosed and rounded frame and by offering physicians the ability to fully recapture and reposition the device during the procedure. Furthermore, the frame of the new device is designed to enhance sealing within the left atrial appendage.
Boston Scientific has begun a limited market release of the WATCHMAN FLX device in Europe and expects to expand commercialization to additional sites in the second half of 2019. The company also plans to begin enrolling European patients in a post-approval registry in the coming months.
In the U.S., the WATCHMAN FLX device is an investigational device and not available for sale.
References
1 "Atrial Fibrillation Fact Sheet." Centers for Disease Control and Prevention. http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm.
2 Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759.
Patients with AF are five times more likely to suffer a stroke than someone with a normal heart rhythm.1 In people with non-valvular atrial fibrillation (AF), data suggests that more than 90 percent of stroke-causing blood clots that come from the heart are formed in the left atrial appendage.2 The WATCHMAN left atrial appendage occlusion devices are intended to reduce the risk of stroke in people with non-valvular AF.
"The WATCHMAN device has been implanted in more than 75,000 patients worldwide and we are pleased that this next-generation technology has been granted European regulatory approval so that we can offer it to patients and clinicians throughout Europe," said Kevin Ballinger, president, Interventional Cardiology, Boston Scientific. "The robust clinical evidence and successful commercial outcomes of the WATCHMAN device to-date reinforce the value of this procedure for all appropriate patients."
The new WATCHMAN FLX device was designed for simplified implantation to fit a wider range of patients, from those with simple to the most complex anatomies. The device allows for implantation flexibility to customize placement with a fully enclosed and rounded frame and by offering physicians the ability to fully recapture and reposition the device during the procedure. Furthermore, the frame of the new device is designed to enhance sealing within the left atrial appendage.
Boston Scientific has begun a limited market release of the WATCHMAN FLX device in Europe and expects to expand commercialization to additional sites in the second half of 2019. The company also plans to begin enrolling European patients in a post-approval registry in the coming months.
In the U.S., the WATCHMAN FLX device is an investigational device and not available for sale.
References
1 "Atrial Fibrillation Fact Sheet." Centers for Disease Control and Prevention. http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm.
2 Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759.