Universitat Mainz02.21.19
Patients of the Mainz University Medical Center with heart valve disease have recently been able to benefit from a new treatment option for a narrowed aortic valve. Since the beginning of January 2019, cardiologists have implanted a new motor-driven and self-expanding heart valve in three patients in Europe for the first time. The advantage: the valve can be implanted more easily and safely, and leakages after implantation are further minimized.
Due to increasing life expectancy, more and more people in Germany are suffering from a symptomatic constriction of the aortic valve. The so-called aortic valve stenosis is the most common heart valve defect of the elderly patient. The standard therapy, especially in younger patients, has been a cardiac-surgical valve replacement. In the last five years, the numbers of catheter-assisted aortic valve replacement therapy (TAVI) have increased tremendously, in particular because of a high proportion of older, partly pre-operated patients. In 2018, there were nearly 20,000 interventions of this kind in Germany. The Department of Cardiology of the Mainz University Medical Center occupies a leading position in the field of minimally invasive heart valve therapy with more than 700 interventions per year. Around 400 TAVI procedures were carried out here last year.
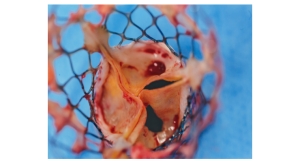
Now cardiologists report the first implantations of the latest model of a self-expanding aortic valve (CENTERA) in Mainz.
The new heart valve has several advantages for patients:
The first three implantations were performed in Mainz by Dr. Ralph Stephan von Bardeleben, Dr. Alexander Tamm, and Professor Andres Beiras-Fernandez on patients aged 73 to 91, two women and one man. Dr. von Bardeleben, head of the Department of Structural Heart Disease and Interventional Heart Valve Therapy, names it "another important step towards a highly accurate and gentle implantation technique that can now largely eliminate potential complications of the procedure in the mostly elderly patients."
"The opportunity to implant the first motor-driven heart valves here in Mainz as Europe's first center is an expression of our great expertise and will help us to further expand our national top position in the field of highly innovative minimally invasive heart valves," commented Professor Thomas Münzel, Director of the Department of Cardiology at the Mainz University Medical Center.
Due to increasing life expectancy, more and more people in Germany are suffering from a symptomatic constriction of the aortic valve. The so-called aortic valve stenosis is the most common heart valve defect of the elderly patient. The standard therapy, especially in younger patients, has been a cardiac-surgical valve replacement. In the last five years, the numbers of catheter-assisted aortic valve replacement therapy (TAVI) have increased tremendously, in particular because of a high proportion of older, partly pre-operated patients. In 2018, there were nearly 20,000 interventions of this kind in Germany. The Department of Cardiology of the Mainz University Medical Center occupies a leading position in the field of minimally invasive heart valve therapy with more than 700 interventions per year. Around 400 TAVI procedures were carried out here last year.
Now cardiologists report the first implantations of the latest model of a self-expanding aortic valve (CENTERA) in Mainz.
The new heart valve has several advantages for patients:
- Its special shape facilitates access to the coronary arteries and reduces leakage near the valve.
- An active bendable catheter system also allows gentle control over the aortic arch. In addition, it is the first heart valve that allows controlled release of the heart valve with a very short nitinol scaffold, an alloy of real titanium and nickel with an electric motor.
The first three implantations were performed in Mainz by Dr. Ralph Stephan von Bardeleben, Dr. Alexander Tamm, and Professor Andres Beiras-Fernandez on patients aged 73 to 91, two women and one man. Dr. von Bardeleben, head of the Department of Structural Heart Disease and Interventional Heart Valve Therapy, names it "another important step towards a highly accurate and gentle implantation technique that can now largely eliminate potential complications of the procedure in the mostly elderly patients."
"The opportunity to implant the first motor-driven heart valves here in Mainz as Europe's first center is an expression of our great expertise and will help us to further expand our national top position in the field of highly innovative minimally invasive heart valves," commented Professor Thomas Münzel, Director of the Department of Cardiology at the Mainz University Medical Center.