Business Wire02.12.19
Intact Vascular Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, has announced its Tack Optimized Balloon Angioplasty II BTK (TOBA II BTK) clinical trial successfully completed enrollment, ahead of schedule. This study further augments Intact Vascular’s robust clinical program and is notably the first pivotal trial investigating a permanent vascular implant in arteries below the knee.
“Patients with critical limb ischemia (CLI) experience painful symptoms and are at increased risk of amputation. Unfortunately, therapeutic options are very limited, and no scaffolding solutions are currently U.S FDA-approved for BTK interventions,” commented George Adams, M.D., M.H.S., director of Cardiovascular and Peripheral Vascular Research, UNC Rex Hospital in Raleigh, N.C., co-principal investigator for the TOBA II BTK study. “The potential to have a treatment option that maintains vessel integrity and improves blood flow will have a significant clinical impact for treating patients with below-the-knee disease.”
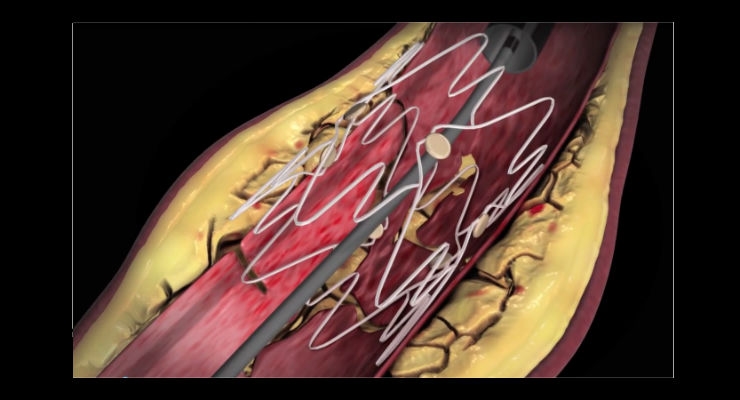
With enrollment completed at 41 U.S. and European sites, the TOBA II BTK trial is a single arm, prospective study to investigate the safety and efficacy of the Tack Endovascular System for the repair of post-angioplasty dissections in the mid/distal popliteal, tibial and peroneal arteries. All patients enrolled (n=233) suffered from CLI, underwent standard balloon angioplasty, and consequently experienced at least one dissection requiring repair.
“Dissections are an expected consequence from balloon angioplasty, yet can have significant implications for patients,” stated Patrick Geraghty, M.D., professor of Surgery and Radiology at the Washington University School of Medicine in St. Louis, Mo., and co-principal investigator for the TOBA II BTK trial. “Early results with the Tack implant are promising, and I look forward to integrating this technology into my below-the-knee treatment algorithm.”
“Reaching full enrollment for our TOBA II BTK study is an important milestone for below-the-knee interventions,” commented Peter Schneider, M.D., vascular surgeon, co-founder and chief medical officer of Intact Vascular. “As the first dissection repair device purpose-built for use in these small vessels, we are excited to provide a solution for patients suffering the painful and debilitating effects of critical limb ischemia and who currently do not have adequate treatment options available. The Tack implant is an adjunct therapy that should diminish the chance that these patients will require an amputation.”
Intact Vascular is a privately held medical device company that develops minimally invasive peripheral vascular products. The Tack Endovascular System is designed to improve peripheral balloon angioplasty results in the treatment of peripheral arterial disease. Pre-loaded with six self-expanding nitinol devices for above-the-knee (ATK) interventions, or four for below-the-knee (BTK) interventions, the Tack Endovascular System can be deployed to treat multiple dissections using a single catheter and leaving behind roughly 70 percent less metal than stents.1 Additionally, the Tack Endovascular System offers low radial force, designed to promote healing, improve outcomes and preserve future treatment options for PAD and critical limb ischemia (CLI) patients.
Intact Vascular is sponsoring three clinical trials to evaluate its Tack Endovascular System: TOBA II, TOBA II BTK and TOBA III. TOBA II is investigating the combination of the Tack implant with plain angioplasty balloons and the BARD Lutonix drug-coated balloon (DCB) in arteries above the knee. TOBA II BTK is investigating the combination of the Tack implant with plain balloon angioplasty in the arteries below the knee and has just completed enrollment. TOBA III has completed enrollment in Europe and is investigating the combination of the Tack implant with the Medtronic IN.PACT Admiral (DCB), inclusive of long lesions.
Reference
1 Bosiers M, Scheinert D, Hendricks JMH et al. Results from the Tack Optimized Balloon Angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. J Vasc Surg 2016;64:109-16.
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.
“Patients with critical limb ischemia (CLI) experience painful symptoms and are at increased risk of amputation. Unfortunately, therapeutic options are very limited, and no scaffolding solutions are currently U.S FDA-approved for BTK interventions,” commented George Adams, M.D., M.H.S., director of Cardiovascular and Peripheral Vascular Research, UNC Rex Hospital in Raleigh, N.C., co-principal investigator for the TOBA II BTK study. “The potential to have a treatment option that maintains vessel integrity and improves blood flow will have a significant clinical impact for treating patients with below-the-knee disease.”
With enrollment completed at 41 U.S. and European sites, the TOBA II BTK trial is a single arm, prospective study to investigate the safety and efficacy of the Tack Endovascular System for the repair of post-angioplasty dissections in the mid/distal popliteal, tibial and peroneal arteries. All patients enrolled (n=233) suffered from CLI, underwent standard balloon angioplasty, and consequently experienced at least one dissection requiring repair.
“Dissections are an expected consequence from balloon angioplasty, yet can have significant implications for patients,” stated Patrick Geraghty, M.D., professor of Surgery and Radiology at the Washington University School of Medicine in St. Louis, Mo., and co-principal investigator for the TOBA II BTK trial. “Early results with the Tack implant are promising, and I look forward to integrating this technology into my below-the-knee treatment algorithm.”
“Reaching full enrollment for our TOBA II BTK study is an important milestone for below-the-knee interventions,” commented Peter Schneider, M.D., vascular surgeon, co-founder and chief medical officer of Intact Vascular. “As the first dissection repair device purpose-built for use in these small vessels, we are excited to provide a solution for patients suffering the painful and debilitating effects of critical limb ischemia and who currently do not have adequate treatment options available. The Tack implant is an adjunct therapy that should diminish the chance that these patients will require an amputation.”
Intact Vascular is a privately held medical device company that develops minimally invasive peripheral vascular products. The Tack Endovascular System is designed to improve peripheral balloon angioplasty results in the treatment of peripheral arterial disease. Pre-loaded with six self-expanding nitinol devices for above-the-knee (ATK) interventions, or four for below-the-knee (BTK) interventions, the Tack Endovascular System can be deployed to treat multiple dissections using a single catheter and leaving behind roughly 70 percent less metal than stents.1 Additionally, the Tack Endovascular System offers low radial force, designed to promote healing, improve outcomes and preserve future treatment options for PAD and critical limb ischemia (CLI) patients.
Intact Vascular is sponsoring three clinical trials to evaluate its Tack Endovascular System: TOBA II, TOBA II BTK and TOBA III. TOBA II is investigating the combination of the Tack implant with plain angioplasty balloons and the BARD Lutonix drug-coated balloon (DCB) in arteries above the knee. TOBA II BTK is investigating the combination of the Tack implant with plain balloon angioplasty in the arteries below the knee and has just completed enrollment. TOBA III has completed enrollment in Europe and is investigating the combination of the Tack implant with the Medtronic IN.PACT Admiral (DCB), inclusive of long lesions.
Reference
1 Bosiers M, Scheinert D, Hendricks JMH et al. Results from the Tack Optimized Balloon Angioplasty (TOBA) study demonstrate the benefits of minimal metal implants for dissection repair after angioplasty. J Vasc Surg 2016;64:109-16.
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.